How Many Valence Electrons Are In F
listenit
Mar 22, 2025 · 5 min read
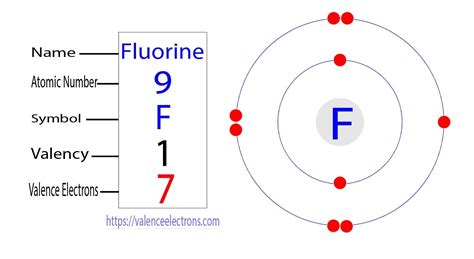
Table of Contents
How Many Valence Electrons are in f-block Elements? Understanding the f-block and its Electronic Configuration
The question "How many valence electrons are in f?" isn't straightforward. Unlike s, p, and d blocks where valence electrons are relatively easy to identify, the f-block (also known as the lanthanide and actinide series) presents a unique challenge. This article delves deep into the complexities of f-block electron configurations, exploring the nuances of valence electrons in these elements and clarifying common misconceptions.
Understanding Valence Electrons
Before diving into the f-block, let's refresh our understanding of valence electrons. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. They determine an element's chemical properties and reactivity. For elements in the s, p, and d blocks, identifying valence electrons is relatively simple. For example:
- s-block: Elements have 1 or 2 valence electrons (e.g., Lithium (Li) has 1, Beryllium (Be) has 2).
- p-block: Elements have 3 to 8 valence electrons (e.g., Boron (B) has 3, Oxygen (O) has 6).
- d-block: The situation is slightly more nuanced. While the outermost s subshell is always filled first, the d subshell electrons also contribute to bonding in many cases, so the number of valence electrons is often considered as the sum of the outermost s and d electrons.
The Complexity of the f-block
The f-block elements, comprising the lanthanides (atomic numbers 57-71) and actinides (atomic numbers 89-103), occupy the inner 4f and 5f orbitals, respectively. This inner positioning significantly impacts how we define "valence electrons."
The core issue is the shielding effect. The 4f and 5f electrons are effectively shielded from the influence of other atoms by the intervening 5s, 5p, 6s, 6p, 7s, and 7p electrons. This shielding weakens the interaction of the f-electrons with other atoms, making their participation in chemical bonding less direct compared to s, p, or even d electrons.
Defining Valence Electrons in f-block Elements: A nuanced perspective
Therefore, a simple count of the f-electrons isn't sufficient to define the number of valence electrons in f-block elements. Instead, a more accurate depiction considers the electrons involved in chemical bonding, which primarily involves the outermost s and d electrons.
The conventional approach considers the outermost s and d electrons as the primary valence electrons for f-block elements. This means that lanthanides typically exhibit a +3 oxidation state, losing their two 6s electrons and one 4f electron. Similarly, actinides also show varying oxidation states, often involving the loss of 7s electrons and 5f electrons.
Oxidation States and Variable Valence: The Reality of f-block Chemistry
The variable oxidation states observed in f-block elements are a testament to the complex interplay of their electrons. While the simplistic view might suggest a fixed number of valence electrons based on the 4f or 5f subshell occupancy, the reality is far more intricate. The energy levels of the 4f and 5f electrons are relatively close to those of the 5d and 6d orbitals, respectively. This proximity allows for multiple electrons to participate in bonding in various combinations.
For example, Cerium (Ce) can exhibit +3 and +4 oxidation states, meaning it can lose three or four electrons to form compounds. This demonstrates that it's not always just the outermost electrons participating in bonding. Similarly, Uranium (U) shows a wider array of oxidation states ranging from +3 to +6.
Contrasting f-block with d-block: A comparative analysis
The situation differs markedly from the d-block elements where the participation of the d-electrons in bonding is more prominent. Although d-electrons are also shielded, their involvement in chemical bonding is generally higher than that of the f-electrons. This is due to their higher energy level and less effective shielding.
The Role of Relativistic Effects
Adding another layer of complexity, relativistic effects become increasingly significant in the heavier actinides. These effects influence the energy levels and orbital sizes, further complicating the simple picture of valence electrons. The relativistic contraction of s and p orbitals and the relativistic expansion of d and f orbitals significantly affect the chemical behavior of these elements.
Practical Applications and Implications
Understanding the subtleties of valence electrons in f-block elements is crucial for various applications. Their unique chemical properties find use in:
- Catalysis: Lanthanides are used as catalysts in various industrial processes due to their variable oxidation states and coordination chemistry.
- Magnets: Certain lanthanide compounds exhibit strong magnetic properties, making them vital components of powerful permanent magnets.
- Lighting: Many lanthanide compounds exhibit vibrant colors when excited, leading to their use in lighting and display technologies.
- Nuclear Technology: Actinides play a critical role in nuclear energy and weaponry. Understanding their chemical behavior is crucial for safe handling and processing.
Conclusion: A nuanced understanding is key
In conclusion, the question of "how many valence electrons are in f?" doesn't have a simple numerical answer. While the f-electrons are present, their involvement in chemical bonding is significantly less direct compared to s, p, and d electrons. The primary valence electrons in f-block elements are considered to be the outermost s and d electrons. The variable oxidation states exhibited by these elements demonstrate the complexity of their electronic configurations and the significant roles of shielding, relativistic effects, and the proximity in energy levels of different subshells. A thorough understanding of this nuanced perspective is essential for comprehending the rich and diverse chemistry of the f-block elements and their applications. Further research continually refines our understanding of these complex elements and their fascinating properties.
Latest Posts
Latest Posts
-
5 To The Negative 1 Power
Mar 23, 2025
-
How Many Neutrons Are In Neon
Mar 23, 2025
-
You Need To Prepare An Acetate Buffer Of Ph
Mar 23, 2025
-
Is Alcohol A Acid And A Base Bronsted
Mar 23, 2025
-
Muscle That Subdivides The Ventral Body Cavity
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In F . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
