How Many Neutrons Are In Neon
listenit
Mar 23, 2025 · 5 min read
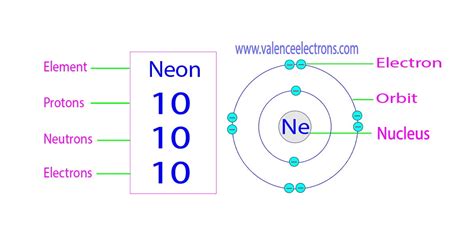
Table of Contents
How Many Neutrons are in Neon? A Deep Dive into Isotopes and Atomic Structure
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, is a fascinating element to study. But beyond its flashy applications, the question of its internal structure, specifically the number of neutrons, offers a glimpse into the complexities of atomic physics. This article will explore the intricacies of neon's neutron count, delving into isotopes, atomic mass, and the significance of this seemingly simple numerical value.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the neutron count of neon, let's refresh our understanding of basic atomic structure. Every atom consists of three fundamental particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; all neon atoms have 10 protons.
- Neutrons: Neutrally charged particles residing alongside protons in the nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom.
The number of protons and neutrons determines the atom's mass number, while the number of protons alone defines its atomic number. Neon's atomic number is 10, meaning all neon atoms possess 10 protons. However, the neutron count isn't fixed.
Isotopes of Neon: The Variable Neutron Count
The key to understanding the varying neutron numbers in neon lies in the concept of isotopes. Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This difference in neutron count affects the atom's mass but not its chemical properties. Neon has three naturally occurring stable isotopes:
- Neon-20 (²⁰Ne): This is the most abundant isotope, accounting for approximately 90.48% of naturally occurring neon. It contains 10 protons and 10 neutrons (20 - 10 = 10 neutrons).
- Neon-21 (²¹Ne): This isotope makes up about 0.27% of naturally occurring neon. It has 10 protons and 11 neutrons (21 - 10 = 11 neutrons).
- Neon-22 (²²Ne): This is the second most abundant isotope, comprising approximately 9.25% of natural neon. It has 10 protons and 12 neutrons (22 - 10 = 12 neutrons).
Therefore, there's no single answer to "how many neutrons are in neon?" The answer depends on the specific isotope.
Calculating Neutron Number: A Simple Formula
Determining the number of neutrons in any isotope is straightforward. Use this simple formula:
Number of neutrons = Mass number - Atomic number
For example:
- Neon-20: 20 (mass number) - 10 (atomic number) = 10 neutrons
- Neon-21: 21 (mass number) - 10 (atomic number) = 11 neutrons
- Neon-22: 22 (mass number) - 10 (atomic number) = 12 neutrons
This formula works for all elements and their isotopes.
The Significance of Isotopic Abundance
The isotopic abundance of neon – the relative percentage of each isotope in a naturally occurring sample – is crucial in several applications. Knowing the abundance of each isotope allows scientists to:
- Calculate the average atomic mass: The average atomic mass of neon (approximately 20.18 amu) is a weighted average of the masses of its isotopes, reflecting their relative abundances.
- Analyze geological processes: Isotopic ratios can serve as tracers in geological studies, helping researchers understand processes like magma formation and mineral formation.
- Understand nuclear reactions: Studying isotopic abundances helps scientists analyze nuclear reactions and the stability of different isotopes.
Neon's Applications: From Lighting to Lasers
Neon's unique properties, stemming from its electronic configuration and the presence of its different isotopes, make it useful in various technological applications:
- Neon lighting: The characteristic reddish-orange glow of neon signs is a result of the excitation of neon atoms by an electric current.
- Helium-neon lasers: Helium-neon lasers, utilizing a mixture of helium and neon gases, produce a coherent beam of red light used in barcode scanners, laser pointers, and research applications.
- Cryogenics: Neon's low boiling point makes it useful as a refrigerant in cryogenic applications, though less commonly than helium or nitrogen.
- High-voltage indicators: Neon's ability to conduct electricity at high voltages makes it useful in high-voltage indicators.
These applications rely on the collective properties of the neon isotopes present in naturally occurring neon gas.
Beyond the Stable Isotopes: Radioactive Neon Isotopes
While neon has three stable isotopes, several radioactive isotopes have been synthesized artificially. These radioactive isotopes have short half-lives and are primarily used in research. Their study provides valuable insights into nuclear physics and decay processes.
Conclusion: Understanding Neon's Neutron Count and its Significance
The number of neutrons in neon isn't a single value; it varies depending on the isotope. Neon-20 has 10 neutrons, Neon-21 has 11, and Neon-22 has 12. Understanding the isotopic composition of neon is crucial for interpreting its physical and chemical properties and for its various applications in science and technology. The study of isotopes highlights the rich diversity within even the simplest elements, offering a fascinating window into the complexities of atomic structure and nuclear physics. The seemingly simple question of "how many neutrons are in neon?" opens up a world of scientific exploration and understanding. From the vibrant glow of neon signs to the precision of helium-neon lasers, the properties of this element, intricately linked to its isotopic composition, continue to shape our modern world. The variations in neutron count not only affect the mass but also contribute to the unique behavior and applications of this remarkable element.
Latest Posts
Latest Posts
-
What Is The Gcf Of 48 And 24
Mar 25, 2025
-
Molarity Of Acetic Acid In Vinegar
Mar 25, 2025
-
How Many Minutes Are In 1 Week
Mar 25, 2025
-
How Many 3rds In A Cup
Mar 25, 2025
-
Can Travel Through A Empty Space
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Neon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
