How Many Valence Electrons Are In Al
listenit
Mar 25, 2025 · 5 min read
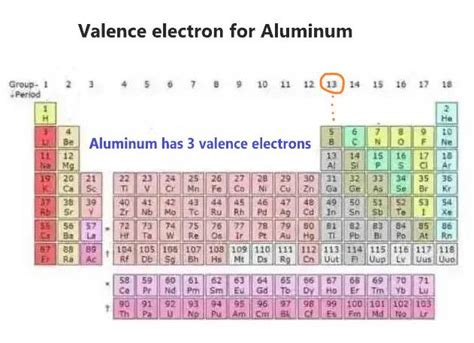
Table of Contents
How Many Valence Electrons Are in Al (Aluminum)? Understanding Valence Electrons and Their Importance
Aluminum (Al), a lightweight yet strong metal, plays a crucial role in various industries, from construction to aerospace. Understanding its atomic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and reactivity. This comprehensive guide will delve into the intricacies of valence electrons, explaining how to determine the number in aluminum and highlighting its significance in chemical bonding and material properties.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and are therefore the primary participants in chemical reactions. They determine an element's reactivity, the types of bonds it can form (ionic, covalent, or metallic), and its overall chemical properties. Think of them as the atom's "social butterflies" – they're the ones interacting with other atoms to form molecules and compounds.
The number of valence electrons an atom possesses is directly related to its position on the periodic table. Specifically, it's determined by the atom's group number (vertical column). This provides a simple and efficient way to predict an element's reactivity and bonding behavior.
Importance of Valence Electrons in Chemical Bonding
Valence electrons are fundamental to understanding chemical bonding. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements). This stability is usually achieved by having a full outermost electron shell, typically containing eight electrons (the octet rule). Atoms achieve this stability by either gaining, losing, or sharing valence electrons with other atoms, forming chemical bonds.
-
Ionic Bonds: In ionic bonds, atoms transfer valence electrons from one atom to another. This results in the formation of ions – positively charged cations (electron loss) and negatively charged anions (electron gain). The electrostatic attraction between these oppositely charged ions forms the ionic bond. For example, aluminum readily loses electrons to form a 3+ cation.
-
Covalent Bonds: In covalent bonds, atoms share valence electrons to achieve a stable electron configuration. This sharing creates a strong bond between the atoms, forming molecules. While less common for aluminum, it can participate in covalent bonding in some specific compounds.
-
Metallic Bonds: In metals like aluminum, valence electrons are delocalized, meaning they are not associated with a particular atom but rather move freely throughout the metal lattice. This "sea" of delocalized electrons is responsible for the characteristic properties of metals, such as high electrical and thermal conductivity, malleability, and ductility.
Determining the Number of Valence Electrons in Aluminum (Al)
Aluminum's atomic number is 13, meaning it has 13 protons and 13 electrons in a neutral atom. To determine its number of valence electrons, we need to consider its electron configuration. Aluminum's electron configuration is 1s²2s²2p⁶3s²3p¹.
The key here is the outermost electron shell. For aluminum, the outermost shell is the third shell (n=3), which contains three electrons (2 in the 3s subshell and 1 in the 3p subshell). Therefore, aluminum has 3 valence electrons.
Using the Periodic Table to Determine Valence Electrons
The periodic table offers a quick way to find the number of valence electrons. Aluminum is in Group 13 (or IIIA) of the periodic table. For the representative elements (Groups 1-18), the group number (excluding the transition metals) usually corresponds to the number of valence electrons. Therefore, aluminum, being in Group 13, has 3 valence electrons. This is a useful shortcut, especially for understanding the chemical behavior of various elements.
Significance of Aluminum's 3 Valence Electrons
Aluminum's three valence electrons have profound consequences for its properties and applications:
-
Reactivity: With three valence electrons, aluminum readily loses these electrons to achieve a stable octet configuration. This makes aluminum relatively reactive, especially with oxidizing agents like oxygen. Its tendency to lose electrons contributes to its corrosion resistance due to the formation of a protective aluminum oxide layer (Al₂O₃).
-
Conductivity: The delocalized valence electrons in aluminum's metallic lattice contribute to its excellent electrical and thermal conductivity. This property is exploited in numerous applications, including electrical wiring and heat sinks.
-
Alloying: Aluminum's ability to form alloys with other metals is largely due to its three valence electrons. These alloys exhibit enhanced properties, such as increased strength, hardness, or corrosion resistance, leading to their extensive use in various industries.
-
Bonding: The three valence electrons allow aluminum to form ionic bonds readily, particularly with nonmetals. Examples include aluminum oxide (Al₂O₃), aluminum chloride (AlCl₃), and aluminum nitride (AlN), all crucial compounds in various applications.
Aluminum's Role in Different Industries
Aluminum's unique properties, dictated by its three valence electrons, have led to its widespread use in numerous industries:
-
Aerospace: Its low density and high strength-to-weight ratio make it ideal for aircraft construction, reducing fuel consumption and improving performance.
-
Automotive: Aluminum alloys are used extensively in car bodies and components due to their lightness, strength, and corrosion resistance, leading to improved fuel efficiency and safety.
-
Construction: Aluminum is used in building materials, such as window frames, doors, and roofing, owing to its durability, weather resistance, and ease of fabrication.
-
Packaging: Aluminum foil and cans are widely used in the food and beverage industry due to their barrier properties against oxygen and moisture, protecting the product's quality and shelf life.
-
Electronics: Aluminum's high electrical conductivity makes it valuable in electronic components, such as printed circuit boards and heat sinks.
Conclusion: The Importance of Valence Electrons in Understanding Aluminum
The number of valence electrons in an atom is a fundamental aspect that dictates its chemical behavior and properties. Aluminum, with its three valence electrons, exemplifies this principle. Understanding the role of these valence electrons is crucial in comprehending aluminum's reactivity, its ability to form bonds, its excellent conductivity, and its widespread use in a vast array of applications. The ability to readily lose these three electrons explains aluminum's metallic properties, its corrosion resistance, and its ability to form strong alloys, making it an indispensable element in modern technology and everyday life. The simple yet profound concept of valence electrons provides a powerful tool to understand the behavior of elements and their impact on our world.
Latest Posts
Latest Posts
-
Using Matrix To Solve System Of Equations
Mar 26, 2025
-
What Percent Of 48 Is 12
Mar 26, 2025
-
What Is The Measure Of X
Mar 26, 2025
-
Balanced Equation For Sodium Hydroxide And Sulphuric Acid
Mar 26, 2025
-
The Density Of Gold Is 19 3 G Cm3
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Al . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
