Balanced Equation For Sodium Hydroxide And Sulphuric Acid
listenit
Mar 26, 2025 · 6 min read
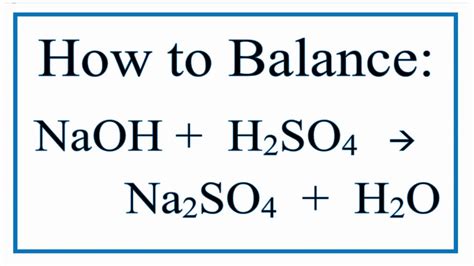
Table of Contents
The Balanced Equation for Sodium Hydroxide and Sulphuric Acid: A Deep Dive
The reaction between sodium hydroxide (NaOH), a strong base, and sulfuric acid (H₂SO₄), a strong acid, is a classic example of a neutralization reaction. Understanding this reaction, including its balanced equation and the stoichiometry involved, is fundamental to chemistry. This article will explore this reaction in detail, covering its balanced equation, the types of reactions involved, practical applications, and safety considerations.
The Balanced Chemical Equation
The reaction between sodium hydroxide and sulfuric acid produces sodium sulfate and water. The unbalanced equation is:
NaOH + H₂SO₄ → Na₂SO₄ + H₂O
This equation is unbalanced because the number of atoms of each element is not equal on both sides of the equation. To balance it, we need to adjust the coefficients (the numbers in front of the chemical formulas) so that the number of each type of atom is the same on both the reactant and product sides. The balanced equation is:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
This balanced equation shows that two moles of sodium hydroxide react with one mole of sulfuric acid to produce one mole of sodium sulfate and two moles of water. This ratio is crucial for understanding the stoichiometry of the reaction and performing calculations involving the reaction's quantities.
Types of Reactions Involved
The reaction between sodium hydroxide and sulfuric acid is primarily a neutralization reaction. This is because an acid (H₂SO₄) reacts with a base (NaOH) to form a salt (Na₂SO₄) and water (H₂O). Neutralization reactions are characterized by the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water.
Beyond neutralization, this reaction can also be classified as a double displacement reaction or metathesis reaction. In this type of reaction, the positive and negative ions of two different compounds switch places to form two new compounds. In this case, the sodium (Na⁺) ion from NaOH switches places with the hydrogen (H⁺) ions from H₂SO₄, resulting in the formation of Na₂SO₄ and H₂O.
Stoichiometric Calculations and Applications
The balanced equation provides the foundation for various stoichiometric calculations. For example, if we know the amount of one reactant, we can determine the amount of other reactants required or the amount of products formed. This is essential in various applications, including:
1. Titration
The reaction between NaOH and H₂SO₄ is frequently used in acid-base titrations. Titration is a quantitative analytical technique used to determine the concentration of an unknown solution (either the acid or the base) by reacting it with a solution of known concentration (the standard solution). By carefully measuring the volume of the standard solution required to neutralize the unknown solution, we can calculate the concentration of the unknown. The stoichiometry of the balanced equation is critical in these calculations. For instance, if we are titrating H₂SO₄ with a standard NaOH solution, the 2:1 mole ratio from the balanced equation dictates that twice the number of moles of NaOH is needed to neutralize the same number of moles of H₂SO₄.
2. Industrial Applications
Sulfuric acid is a cornerstone chemical in many industrial processes. Its reaction with sodium hydroxide might be used in:
- Wastewater Treatment: Neutralizing acidic wastewater streams from industrial processes. Adding a calculated amount of NaOH can adjust the pH to acceptable levels, reducing environmental impact.
- Chemical Synthesis: Although less common than other reactions involving sulfuric acid, the controlled neutralization with NaOH could be a step in some specialized chemical syntheses.
- pH Control: In various industrial processes requiring precise pH control, the addition of NaOH to a sulfuric acid solution can be used to carefully adjust the pH to the desired level.
3. Laboratory Applications
In the laboratory, the reaction can be used for:
- Preparing Standard Solutions: Precisely neutralizing a sulfuric acid solution with a known amount of NaOH can be used in preparing a standard sulfuric acid solution for titrations or other experiments.
- Demonstrations: The reaction serves as a good demonstration of acid-base neutralization and stoichiometry in chemistry education. The heat released during the reaction (exothermic reaction) can be easily observed.
Safety Precautions
When handling sodium hydroxide and sulfuric acid, stringent safety precautions must be observed:
- Eye Protection: Always wear safety goggles or a face shield to protect your eyes from splashes.
- Gloves: Use chemical-resistant gloves to protect your skin from contact with these corrosive chemicals.
- Lab Coat: Wear a lab coat to protect your clothing.
- Ventilation: Perform the reaction in a well-ventilated area or under a fume hood to avoid inhaling any fumes. The reaction can generate heat and potentially release some spray during mixing.
- Proper Disposal: Dispose of the resulting solution according to your institution's or local regulations for chemical waste. Never pour them down the drain without neutralization and proper disposal protocol.
- Slow Addition: Always add the acid to the base slowly and carefully, stirring continuously. Adding the base to the acid can cause a violent exothermic reaction, generating significant heat and possibly splashing.
Factors Affecting the Reaction Rate
Several factors can influence the rate of the neutralization reaction:
- Concentration: Higher concentrations of both reactants generally lead to a faster reaction rate due to increased collision frequency between reactant molecules.
- Temperature: Increasing the temperature increases the kinetic energy of the molecules, leading to more frequent and energetic collisions, thereby increasing the reaction rate.
- Stirring: Stirring the reaction mixture ensures uniform mixing and increases the frequency of collisions between reactant molecules, leading to a faster reaction rate.
- Surface Area: While not directly relevant in this homogeneous reaction (both reactants are in aqueous solution), the concept applies to reactions involving solid reactants. A larger surface area facilitates faster reaction rates.
Beyond the Basics: Understanding the Ions
The reaction's essence lies in the interaction of ions. Sulfuric acid, a diprotic acid, dissociates in water to produce hydrogen ions (H⁺) and bisulfate ions (HSO₄⁻):
H₂SO₄ → H⁺ + HSO₄⁻
The bisulfate ion can further dissociate, though to a lesser extent:
HSO₄⁻ ⇌ H⁺ + SO₄²⁻
Sodium hydroxide dissociates completely in water to produce sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH → Na⁺ + OH⁻
The neutralization reaction involves the combination of H⁺ ions from the sulfuric acid and OH⁻ ions from the sodium hydroxide to form water:
H⁺ + OH⁻ → H₂O
The sodium ions (Na⁺) and sulfate ions (SO₄²⁻) remain in solution as spectator ions, meaning they don't participate directly in the reaction but are present in the final product, sodium sulfate (Na₂SO₄).
Conclusion
The reaction between sodium hydroxide and sulfuric acid is a fundamental chemical reaction with wide-ranging applications in various fields. Understanding its balanced equation, stoichiometry, and safety precautions is crucial for anyone working with these chemicals. The reaction serves as an excellent example of acid-base neutralization, double displacement reactions, and the importance of stoichiometric calculations in chemistry. Remember always to prioritize safety when conducting any chemical experiments. By carefully considering the factors that affect the reaction rate and following the appropriate safety protocols, you can safely and effectively utilize this important chemical reaction.
Latest Posts
Latest Posts
-
Covalent Bonds Hold Atoms Together Because They
Mar 29, 2025
-
What Does Ate Mean In Chemistry
Mar 29, 2025
-
Standard Enthalpy Of Formation Of Ethanol
Mar 29, 2025
-
Do Parallelograms Have 4 Right Angles
Mar 29, 2025
-
Least Common Multiple Of 10 And 8
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For Sodium Hydroxide And Sulphuric Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
