How Many Valance Electrons Does Iron Have
listenit
Mar 23, 2025 · 5 min read
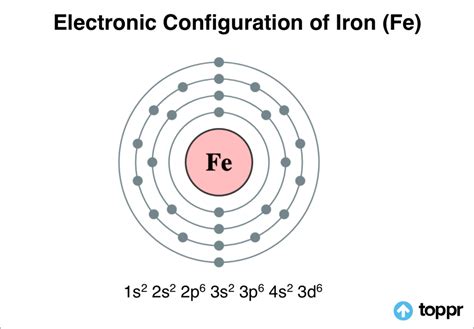
Table of Contents
How Many Valence Electrons Does Iron Have? Understanding Electronic Configuration and Chemical Behavior
Iron, a ubiquitous element vital to life and industry, presents an intriguing case study in electron configuration and chemical behavior. Understanding its valence electrons is key to comprehending its diverse properties and reactivity. This comprehensive article delves into the intricacies of iron's electronic structure, exploring its valence electron count, the implications for its bonding characteristics, and its resulting impact across various scientific and technological domains.
Defining Valence Electrons: The Key to Reactivity
Before we dive into iron's specific electron configuration, let's establish a clear understanding of valence electrons. These are the electrons located in the outermost shell of an atom, also known as the valence shell. They are the primary participants in chemical bonding, determining an element's reactivity and the types of chemical bonds it can form. The number of valence electrons dictates an element's position in the periodic table and profoundly influences its chemical properties.
The Significance of the Outermost Shell
The outermost shell's electrons experience weaker attraction from the positively charged nucleus compared to inner-shell electrons. This makes them more readily available to interact with other atoms, forming bonds and creating molecules or ionic compounds. Atoms strive for stability, often achieving it by filling their outermost shell – a concept central to understanding chemical bonding.
Iron's Electronic Configuration: Unveiling the Mystery
Iron (Fe), with an atomic number of 26, possesses 26 electrons. To determine its valence electrons, we must delve into its electronic configuration. This describes how electrons are distributed among the various energy levels and sublevels within the atom.
Orbital Filling and Electron Configuration
Following the Aufbau principle, electrons fill orbitals in order of increasing energy. The electronic configuration of iron is: 1s²2s²2p⁶3s²3p⁶4s²3d⁶.
-
1s², 2s², 2p⁶, 3s², 3p⁶: These inner shells are completely filled and are considered core electrons. They are tightly bound to the nucleus and do not typically participate in chemical bonding.
-
4s²3d⁶: These are the valence electrons. Note that even though the 3d subshell is lower in energy than the 4s subshell, the 4s electrons are generally considered valence electrons because they are outermost and involved in bonding.
The Ambiguity of Iron's Valence Electrons: A Deeper Dive
While the simplistic view might suggest iron has two valence electrons (from the 4s subshell), the reality is more nuanced. The 3d electrons are also involved in chemical bonding, particularly in transition metal chemistry. Therefore, iron exhibits variable valency, meaning it can lose different numbers of electrons to form ions with various charges.
Variable Oxidation States: A Hallmark of Transition Metals
Iron's variable oxidation states are a direct consequence of its partially filled 3d subshell. Common oxidation states for iron include +2 (ferrous) and +3 (ferric).
-
Fe²⁺ (ferrous): In this ion, iron loses two electrons, typically from the 4s subshell. This leaves the 3d subshell with six electrons.
-
Fe³⁺ (ferric): In this ion, iron loses three electrons – two from the 4s subshell and one from the 3d subshell. This leaves the 3d subshell with five electrons.
The ability of iron to adopt multiple oxidation states contributes to its diverse chemistry and its ability to form a wide range of compounds.
Implications for Chemical Bonding: A Versatile Element
The variable valence of iron profoundly influences the types of chemical bonds it forms. Iron can participate in various bonding scenarios:
Ionic Bonding: The Transfer of Electrons
When iron forms ionic compounds, it typically loses electrons to achieve a stable electron configuration. The resulting positively charged iron ion (cation) then interacts electrostatically with negatively charged anions, forming an ionic bond. Examples include iron oxides (FeO, Fe₂O₃) and iron sulfides (FeS).
Covalent Bonding: Sharing Electrons
While less common than ionic bonding, iron can also participate in covalent bonding, where electrons are shared between atoms. This is particularly evident in organometallic compounds, where iron forms bonds with carbon-containing ligands.
Metallic Bonding: A Sea of Electrons
In metallic iron, the valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged iron ions. This delocalization accounts for iron's high electrical and thermal conductivity, malleability, and ductility.
Iron's Role in Biology and Industry: A Multifaceted Element
Iron's unique electronic configuration and consequent chemical properties make it indispensable in various biological and industrial processes.
Biological Significance: Essential for Life
Iron is an essential element in living organisms, playing crucial roles in various biological processes. It is a key component of hemoglobin, the protein responsible for oxygen transport in the blood. It also features in numerous enzymes involved in vital metabolic pathways. The precise number of valence electrons and iron's ability to change oxidation states are critical for its function in these biological systems.
Industrial Applications: A Cornerstone of Modern Technology
Iron's strength, durability, and relatively low cost have made it a cornerstone of modern industry. It is extensively used in the production of steel, which is a crucial material in construction, transportation, and manufacturing. Iron's ability to form alloys with other metals enhances its properties, creating materials with specific characteristics tailored to various applications. Furthermore, iron compounds are utilized in various industries, including pigments, catalysts, and magnetic materials.
Conclusion: A Comprehensive Understanding of Iron's Valence Electrons
While a simplified answer might suggest iron has two valence electrons, a deeper understanding reveals a more complex picture. The participation of both 4s and 3d electrons in chemical bonding leads to iron's variable valency, which is the cornerstone of its rich and diverse chemistry. This variable valency is crucial for iron's biological roles and its widespread industrial applications. From its role in oxygen transport in hemoglobin to its use in the construction of skyscrapers, iron's unique electronic structure profoundly influences its behavior and makes it an element vital to life and technology.
This comprehensive analysis highlights the importance of understanding not only the simple count of valence electrons but also the intricacies of electronic configuration and its impact on an element's chemical behavior. The seemingly simple question of "how many valence electrons does iron have?" opens a window into a vast and fascinating world of chemical principles and their far-reaching consequences. This understanding is crucial for anyone seeking a deeper appreciation of chemistry and its significance in the world around us.
Latest Posts
Latest Posts
-
Is State Of Matter A Physical Change
Mar 24, 2025
-
What Is The Square Root Of 244
Mar 24, 2025
-
How To Find Molarity From Titration
Mar 24, 2025
-
Is Changing Color A Chemical Change
Mar 24, 2025
-
What Is The Change In Velocity Over Time
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Valance Electrons Does Iron Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
