How Many Protons Does Magensiuum Ion Have
listenit
Mar 21, 2025 · 6 min read
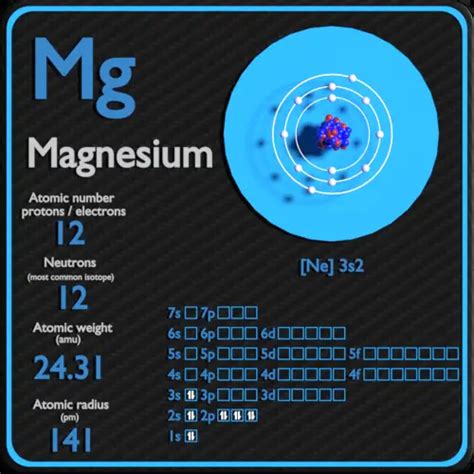
Table of Contents
How Many Protons Does a Magnesium Ion Have? Understanding Atomic Structure and Ions
Magnesium, a vital element for human health and various industrial applications, presents an excellent case study for understanding atomic structure and ionic behavior. This article delves deep into the question: how many protons does a magnesium ion have? We'll explore the core concepts of atomic number, isotopes, ions, and their implications for magnesium's properties and reactivity.
Understanding Atomic Structure: The Foundation
Before addressing the magnesium ion specifically, let's establish a firm understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The atomic number is the defining characteristic of an element. It represents the number of protons in the nucleus of an atom. This number is unique to each element and is found on the periodic table. For instance, hydrogen has an atomic number of 1 (one proton), helium has 2, and so on.
Magnesium's Atomic Structure: A Closer Look
Magnesium (Mg), located in Group 2 (alkaline earth metals) of the periodic table, has an atomic number of 12. This unequivocally means that a neutral magnesium atom possesses 12 protons.
This is fundamental. The number of protons never changes for a given element. It is what makes magnesium, magnesium. Adding or removing protons transforms the atom into a completely different element.
A neutral magnesium atom also contains 12 electrons, balancing the positive charge of the 12 protons. These electrons are arranged in electron shells: two in the first shell, and eight in the second shell. The remaining two electrons occupy the third shell. This electron configuration is crucial in determining magnesium's chemical reactivity.
Ions: The Charged Particles
An ion is an atom or molecule that carries a net electrical charge. This charge arises from an imbalance in the number of protons and electrons.
- Cations: Positively charged ions formed when an atom loses electrons.
- Anions: Negatively charged ions formed when an atom gains electrons.
Magnesium is a metal, and metals tend to lose electrons to achieve a more stable electron configuration, typically resembling the nearest noble gas (in this case, Neon).
Magnesium Ion (Mg²⁺): Losing Electrons
Magnesium readily loses its two outermost electrons (valence electrons) to become a magnesium ion (Mg²⁺). This process is called ionization.
Crucially, losing electrons does not affect the number of protons. The magnesium ion still has 12 protons. Only the number of electrons has changed; it now has 10 electrons (12 protons - 2 electrons = +2 charge). This +2 charge represents the ion's overall positive charge.
The stable electron configuration achieved after losing these two electrons makes the magnesium ion chemically stable and less reactive than the neutral magnesium atom. This is a key principle in chemical bonding and reactivity.
Isotopes and their Impact on Mass, Not Charge
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means isotopes have the same atomic number but different mass numbers (the sum of protons and neutrons).
Magnesium has several naturally occurring isotopes, including Magnesium-24 (¹²Mg), Magnesium-25 (²⁵Mg), and Magnesium-26 (²⁶Mg). These isotopes differ in the number of neutrons, affecting their mass, but they all have 12 protons and therefore remain magnesium. The ionic behavior—the loss of two electrons to form Mg²⁺—remains consistent across all magnesium isotopes.
Magnesium's Role in Biology and Industry: A Consequence of its Properties
The properties of magnesium, both as a neutral atom and as an ion, are central to its roles in various biological and industrial processes:
Biological Significance:
- Enzyme Activation: Magnesium ions (Mg²⁺) are crucial cofactors for many enzymes, assisting in various metabolic reactions within the body. These enzymes play vital roles in energy production, DNA replication, and muscle contraction.
- Bone Structure: Magnesium is a key component of bone structure, contributing to bone strength and density.
- Nerve and Muscle Function: Magnesium plays a role in nerve impulse transmission and muscle contraction. Appropriate magnesium levels are essential for maintaining healthy nerve and muscle function.
Industrial Applications:
- Alloying Agent: Magnesium's lightweight nature and ability to form alloys with other metals makes it valuable in various industries, including aerospace and automotive. Magnesium alloys offer strength and lightweight properties desirable in these sectors.
- Reducing Agent: Magnesium's high reactivity allows it to act as a reducing agent in various chemical processes, for example, in the extraction of certain metals from their ores.
- Photography: Magnesium is used in flash photography due to its ability to burn brightly and produce intense light.
Summary: Protons Define the Element, Ions Define Reactivity
Let's reiterate the central point: a magnesium ion (Mg²⁺) possesses 12 protons. This is non-negotiable; it's what makes it magnesium. The +2 charge reflects the loss of two electrons, not a change in the number of protons. The number of neutrons might vary (isotopes), impacting its mass, but not its elemental identity or its ionic charge. Understanding this fundamental principle is crucial to comprehending the properties and diverse applications of magnesium, from its biological roles to its industrial uses. The constancy of the proton number while electron configuration changes highlight the core principles of atomic structure and the formation of ions. This knowledge forms the cornerstone of chemistry and is pivotal in countless scientific fields.
Further Exploration: Beyond the Basics
This exploration of magnesium's atomic structure and ionic behavior provides a strong foundation for delving further into related topics:
- Chemical Bonding: Investigate how magnesium ions interact with other ions and atoms to form various chemical compounds.
- Electrochemistry: Explore the role of magnesium ions in electrochemical processes, such as batteries and corrosion.
- Spectroscopy: Learn how spectroscopic techniques can be used to identify and quantify magnesium ions in different samples.
- Advanced Materials: Investigate the ongoing research into the development of new magnesium-based materials with enhanced properties for various applications.
By understanding the fundamental principles discussed here, you gain a powerful tool to further your knowledge of chemistry and its impact on the world around us. The seemingly simple question of how many protons are in a magnesium ion opens a gateway to a deep understanding of atomic structure, ionic behavior, and the fascinating properties of elements.
Latest Posts
Latest Posts
-
Do You Have To Add Water To Acid
Mar 28, 2025
-
What Is The Fraction Of 70
Mar 28, 2025
-
Light Is A Transverse Or Longitudinal Wave
Mar 28, 2025
-
What Are Chromosomes Called When They Look Like Xs
Mar 28, 2025
-
X 3 X 2 X Factor
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Magensiuum Ion Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
