Do You Have To Add Water To Acid
listenit
Mar 28, 2025 · 5 min read
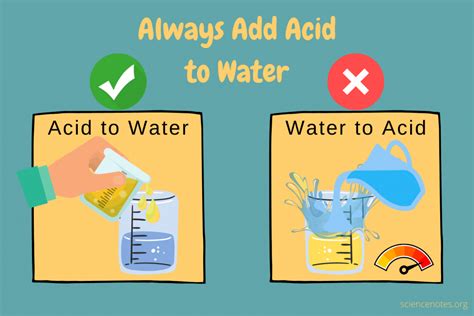
Table of Contents
Do You Have to Add Water to Acid? A Comprehensive Guide to Safe Acid Dilution
The phrase "add acid to water, never water to acid" is a cornerstone of chemistry safety. But why? Understanding the underlying principles behind this crucial rule is paramount for anyone handling acids, from seasoned chemists to hobbyists conducting simple experiments. This comprehensive guide will delve deep into the reasons behind this safety precaution, explore the consequences of improper dilution, and provide practical tips for safe acid handling.
The Exothermic Nature of Acid Dilution
The core reason for adding acid to water, and not vice versa, lies in the exothermic nature of the dilution process. When an acid dissolves in water, it releases a significant amount of heat. This heat is a byproduct of the chemical reaction between the acid molecules and water molecules. This heat generation is known as the heat of dilution.
Understanding Heat of Dilution
The heat of dilution varies significantly depending on the acid's concentration and the amount of water used. Strong acids, such as sulfuric acid (H₂SO₄) and hydrochloric acid (HCl), generate considerably more heat than weaker acids. The concentration of the acid directly impacts the intensity of the exothermic reaction. A more concentrated acid will release proportionally more heat.
The Dangers of Incorrect Dilution
Imagine adding water to concentrated sulfuric acid. The water, being less dense, will initially sit on top of the acid. The heat generated by the reaction will be concentrated at the point of contact between the water and the acid. This localized heating can cause the water to boil violently, resulting in a dangerous ejection of hot acid. The boiling water can also splash concentrated acid, creating a significant risk of burns and other injuries.
This violent reaction is far less likely to occur when acid is added slowly to water. The heat generated is dispersed more evenly throughout the larger volume of water, mitigating the risk of localized boiling and splashing.
Specific Examples: The Perils of Improper Dilution
Let's consider some specific examples to illustrate the dangers of adding water to acid:
Sulfuric Acid (H₂SO₄)
Sulfuric acid is a particularly potent example. It's a highly corrosive and viscous liquid that generates significant heat upon dilution. Adding water to concentrated sulfuric acid can lead to a dramatic, potentially explosive, reaction. The heat generated can cause the mixture to boil and splatter, resulting in severe burns.
Hydrochloric Acid (HCl)
Hydrochloric acid, while less viscous than sulfuric acid, also generates substantial heat upon dilution. While the reaction may not be as explosive as with sulfuric acid, improper dilution can still lead to splashing and burns.
Nitric Acid (HNO₃)
Nitric acid reacts differently than sulfuric or hydrochloric acid. While it also releases heat during dilution, the reaction can produce toxic fumes. Adding water incorrectly increases the risk of inhaling these harmful fumes, posing significant health risks.
Safety Precautions: Mastering the Art of Acid Dilution
Safe acid dilution requires careful planning and execution. Here's a breakdown of the essential safety measures:
1. Personal Protective Equipment (PPE): Your First Line of Defense
Always wear appropriate personal protective equipment (PPE) before handling acids. This includes:
- Safety goggles: Protect your eyes from splashes.
- Lab coat: Protect your clothing and skin.
- Gloves: Choose chemical-resistant gloves appropriate for the specific acid being handled. Nitrile gloves are a common choice.
- Closed-toe shoes: Protect your feet in case of spills.
2. The Dilution Process: Slow and Steady Wins the Race
The correct procedure is crucial. Follow these steps:
- Use a suitable container: Choose a heat-resistant container made of borosilicate glass or a suitable plastic.
- Add the acid slowly: Pour the acid into a larger volume of water, stirring gently but constantly. Never add water to the acid.
- Control the temperature: Use an ice bath or other cooling method to control the temperature rise, especially for large dilutions or highly concentrated acids.
- Always stir: Constant stirring ensures even distribution of heat and prevents localized heating.
- Work in a well-ventilated area: To minimize the risk of inhaling harmful fumes, especially when working with nitric acid.
3. Post-Dilution Handling: Disposal and Storage
Once diluted, acids should be handled with continued care. Follow these steps:
- Label appropriately: Clearly label the diluted acid with its concentration and identity.
- Store safely: Store the diluted acid in a securely capped container, away from incompatible substances.
- Dispose of responsibly: Follow your institution's or local guidelines for the safe disposal of acids.
Beyond the Basics: Addressing Common Concerns
While the "acid to water" rule is fundamental, several related aspects need clarification:
Dilution of Different Acids: Variations in Procedures
The specific dilution procedure may vary slightly depending on the acid. Some acids, like perchloric acid (HClO₄), require special handling and dilution protocols due to their unique properties. Always consult the Safety Data Sheet (SDS) for the specific acid you are handling.
Importance of Slow Addition: Avoiding Localized Heating
Slow and controlled addition is paramount. Rapid addition creates high localized concentrations of acid and water, leading to excessive heat generation and potential hazards.
Cooling Methods: Maintaining a Safe Temperature
Cooling is especially important when diluting concentrated acids. Ice baths, cold water baths, or even running tap water can help regulate the temperature during the dilution process.
Emergency Response: Preparedness for Accidents
Always have a plan in place for accidental spills or other emergencies. Knowing the location of safety showers, eyewash stations, and spill kits is essential.
Conclusion: Safety First, Always
The seemingly simple act of diluting an acid carries significant safety implications. Understanding the exothermic nature of the process and following the established safety precautions is not just good practice; it's essential for preventing serious injury and ensuring a safe working environment. Remember, the principle of "add acid to water, never water to acid" is a fundamental rule, and strictly adhering to it is crucial for protecting yourself and others from potential harm. Always prioritize safety and consult relevant safety data sheets and guidelines before handling any chemicals.
Latest Posts
Latest Posts
-
What Is The Gcf Of 42 And 24
Mar 31, 2025
-
The Least Common Multiple Of 6 And 9
Mar 31, 2025
-
6 1 2 As An Improper Fraction
Mar 31, 2025
-
How Do Sedimentary Rocks Change Into Igneous Rocks
Mar 31, 2025
-
The Sum Of 3 Consecutive Integers
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Do You Have To Add Water To Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
