How Many Protons Do Silver Have
listenit
Mar 28, 2025 · 5 min read
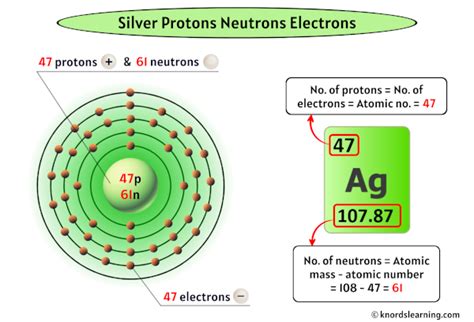
Table of Contents
How Many Protons Does Silver Have? Delving into Atomic Structure and Isotopes
Silver, a lustrous white metal prized for its beauty and conductivity, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of protons it possesses, is key to grasping its chemical properties and behavior. This comprehensive article will explore the answer to the question, "How many protons does silver have?", delving into the concept of atomic number, isotopes, and the significance of this fundamental characteristic in determining silver's unique properties.
Understanding Atomic Number and Protons
The atomic number of an element is arguably its most fundamental characteristic. It represents the number of protons found in the nucleus of a single atom of that element. Protons, along with neutrons, make up the atom's nucleus, while electrons orbit the nucleus in shells. The number of protons uniquely identifies an element; no two elements have the same number of protons.
For silver (Ag), the atomic number is 47. This unequivocally means that every silver atom contains 47 protons. This is a constant; it doesn't change regardless of the form of silver (solid, liquid, or as a compound). This fundamental characteristic is crucial in defining silver's chemical behavior and its position on the periodic table.
Isotopes: Variations in Neutron Count
While the number of protons remains constant for a given element, the number of neutrons can vary. These variations are known as isotopes. Isotopes of the same element have the same number of protons but differ in their number of neutrons. This difference in neutron count affects the atom's mass but not its chemical properties significantly.
Silver has two naturally occurring stable isotopes:
-
Silver-107 (¹⁰⁷Ag): This isotope accounts for approximately 51.84% of naturally occurring silver. It contains 47 protons and 60 neutrons (47 + 60 = 107).
-
Silver-109 (¹⁰⁹Ag): This isotope makes up roughly 48.16% of naturally occurring silver. It also has 47 protons but contains 62 neutrons (47 + 62 = 109).
The mass number (the sum of protons and neutrons) distinguishes these isotopes. While their chemical properties are almost identical, their slightly different masses have implications in certain applications, particularly in areas like mass spectrometry and nuclear physics.
Silver's Properties and the Role of Protons
The 47 protons in silver's nucleus are crucial in determining its physical and chemical properties. These properties make silver a highly valuable and versatile metal:
-
Excellent Conductivity: Silver's electronic configuration, driven by the number of protons and electrons, leads to its exceptional electrical and thermal conductivity. This makes it ideal for use in electronics, electrical contacts, and heat exchangers.
-
High Reflectivity: Silver's unique electronic structure also contributes to its remarkable reflectivity, making it a popular choice for mirrors and other optical applications. Its ability to reflect light efficiently is a direct consequence of its atomic structure.
-
Malleability and Ductility: Silver is easily shaped and drawn into wires due to its metallic bonding, which is in turn a result of the interaction of its electrons, influenced by the presence of 47 protons.
-
Antimicrobial Properties: Silver ions (Ag⁺) exhibit potent antimicrobial properties. This is linked to the chemical reactivity of silver, which is directly influenced by its atomic structure and electron configuration determined by its 47 protons.
Silver in Different Chemical Environments
The 47 protons dictate silver's reactivity and its behavior in different chemical environments. Silver can form various compounds, often exhibiting a +1 oxidation state, reflecting its tendency to lose one electron from its outermost shell to achieve a stable electron configuration. The strong attraction of the nucleus (with its 47 protons) to the electrons shapes its bonding behavior with other elements.
Applications Leveraging Silver's Properties
The unique characteristics of silver, arising directly from its atomic structure and specifically its 47 protons, lead to its widespread use in diverse fields:
-
Electronics: In circuits, contacts, and conductive inks due to its exceptional electrical conductivity.
-
Photography: Historically in photographic films and prints, utilizing its light sensitivity.
-
Jewelry and Ornamental Purposes: Because of its luster, malleability, and resistance to corrosion.
-
Medicine: In wound dressings and antimicrobial coatings thanks to its antimicrobial properties.
-
Catalysis: In various chemical processes, due to its catalytic activity.
Beyond Stable Isotopes: Radioactive Isotopes of Silver
While ¹⁰⁷Ag and ¹⁰⁹Ag are the prevalent naturally occurring isotopes, several radioactive isotopes of silver also exist. These radioactive isotopes are characterized by instability and undergo radioactive decay, emitting particles or energy in the process. The number of protons (47) remains constant, but the number of neutrons varies, influencing the decay mode and half-life of these radioactive isotopes. These radioactive isotopes find applications in nuclear medicine and scientific research.
Conclusion: The Significance of 47 Protons
The answer to "How many protons does silver have?" is definitively 47. This seemingly simple number is crucial in understanding the entirety of silver's properties, behavior, and applications. Its atomic number, the bedrock of its atomic structure, determines its place on the periodic table, governs its chemical reactions, and dictates its unique physical attributes. From its excellent conductivity to its antimicrobial properties, silver's characteristics are a direct consequence of the 47 protons at its core, making it a fascinating and valuable element.
Further Exploration: Isotopic Abundance and Mass Spectrometry
Understanding isotopic abundance—the relative proportion of different isotopes in a naturally occurring sample—requires techniques like mass spectrometry. Mass spectrometry separates isotopes based on their mass-to-charge ratio, allowing scientists to determine the precise abundance of ¹⁰⁷Ag and ¹⁰⁹Ag in a given silver sample. This information is valuable in various scientific fields, including geochemistry, environmental science, and materials science.
The Periodic Table and Silver's Position
Silver's position in the periodic table, Group 11 and Period 5, reflects its electronic configuration, which is directly related to its 47 protons. Its electron configuration influences its reactivity and bonding behavior, explaining its ability to form various compounds and alloys. Studying the periodic table helps us understand the relationships between elements and predict their properties based on their position and atomic number.
This detailed analysis demonstrates the profound impact of a seemingly simple number – the number of protons – in shaping the properties and applications of a fascinating element like silver. The 47 protons within each silver atom are the fundamental key to unlocking its unique characteristics and its significant role in various aspects of modern science and technology.
Latest Posts
Latest Posts
-
Where Is Most Freshwater Found On Earth
Mar 31, 2025
-
Simplify The Square Root Of 512
Mar 31, 2025
-
2 1 6 As An Improper Fraction
Mar 31, 2025
-
Explain One Major Difference Between Purines And Pyrimidines
Mar 31, 2025
-
What Percent Is 1 Out Of 20
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Do Silver Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
