How Many Pi Electrons In A Double Bond
listenit
Apr 01, 2025 · 5 min read
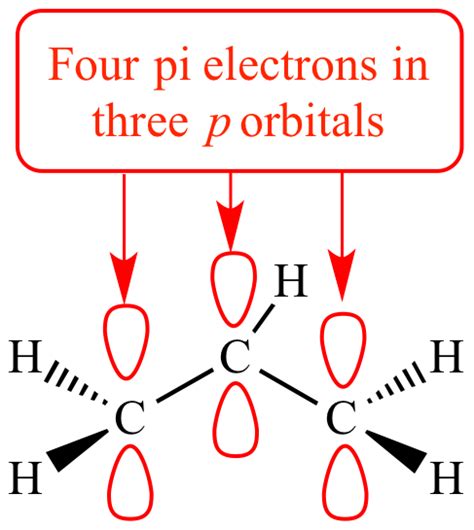
Table of Contents
How Many Pi Electrons in a Double Bond? A Deep Dive into Bonding and Aromaticity
Understanding the number of pi electrons in a double bond is fundamental to grasping organic chemistry concepts like bonding, stability, and aromaticity. This article will delve deep into this topic, explaining the basics, exploring examples, and demonstrating its importance in predicting molecular behavior.
Understanding Pi Bonds and Sigma Bonds
Before we address the central question, let's clarify the types of bonds involved in a double bond. A double bond consists of two distinct bonds: a sigma (σ) bond and a pi (π) bond.
Sigma Bonds (σ Bonds): The Foundation
Sigma bonds are formed by the head-on overlap of atomic orbitals. This overlap is strong and results in a stable, single bond. Think of it as the "backbone" of the bond. In a double bond, one of the bonds is always a sigma bond.
Pi Bonds (π Bonds): Adding Extra Strength and Reactivity
Pi bonds are formed by the sideways overlap of p orbitals. This overlap is weaker than the sigma bond overlap because the electron density is located above and below the internuclear axis (the line connecting the two bonded atoms). This weaker interaction accounts for the lower bond energy of pi bonds compared to sigma bonds. Crucially, it is this pi bond that contributes to the unique properties of double bonds.
The Key Answer: One Pi Bond, Two Pi Electrons
Now, to answer the core question: a double bond contains only one pi bond, and therefore, two pi electrons. These two electrons are found in the pi bonding molecular orbital formed by the sideways overlap of the p orbitals.
This seemingly simple answer forms the basis for understanding more complex concepts in organic chemistry.
Exploring Examples: Visualizing Pi Electrons
Let's illustrate this with some common examples.
Ethene (C₂H₄): The Simplest Example
Ethene, the simplest alkene (a hydrocarbon with a carbon-carbon double bond), provides a clear picture. Each carbon atom in ethene uses one of its p orbitals to form the pi bond with the other carbon atom. Each p orbital contributes one electron, leading to a total of two pi electrons shared in the pi bonding molecular orbital. The remaining electrons form sigma bonds and are not relevant to this particular discussion.
Other Alkenes: Extending the Concept
The principle remains consistent across all alkenes. Regardless of the size or complexity of the alkene molecule, each double bond contributes one pi bond and therefore two pi electrons. For instance, a molecule with two double bonds will have four pi electrons (two pi bonds) in total.
The Importance of Pi Electrons: Aromatics and Reactivity
The presence and distribution of pi electrons significantly influence a molecule's properties, particularly its reactivity and stability.
Aromaticity: A Special Case of Pi Electron Stability
Aromatic compounds, exemplified by benzene, exhibit exceptional stability. Hückel's rule dictates that planar, cyclic, conjugated systems containing (4n + 2) pi electrons, where n is a non-negative integer, are aromatic. This is because these pi electrons are delocalized across the entire ring, creating a particularly stable electron cloud. Benzene, with its six pi electrons (n=1), perfectly exemplifies this rule.
Reactivity: Electrophilic and Nucleophilic Attacks
The pi electrons in double bonds are relatively loosely held compared to sigma electrons. This makes them susceptible to attack by electrophiles (electron-deficient species) in electrophilic addition reactions. The pi electrons can act as a nucleophile, donating electron density to the electrophile and forming a new bond.
Beyond Double Bonds: Conjugated Systems and Extended Pi Systems
The concept of pi electrons extends beyond simple double bonds to encompass conjugated systems. A conjugated system is a molecule where alternating single and multiple bonds allow for the delocalization of pi electrons over more than two atoms.
Conjugated Dienes: Delocalized Pi Electrons
Conjugated dienes, such as 1,3-butadiene, possess two double bonds separated by a single bond. The pi electrons in these double bonds are not localized to a single bond but are delocalized across the entire conjugated system. This delocalization contributes to the molecule's stability and unique reactivity. In 1,3-butadiene, there are four pi electrons in a conjugated system.
Extended Pi Systems: Polymers and Larger Molecules
The principle of delocalized pi electrons extends to larger molecules and polymers. In polyenes with multiple conjugated double bonds, the pi electrons are delocalized across the entire conjugated system. This delocalization leads to different spectroscopic properties and altered reactivity compared to isolated double bonds.
Advanced Concepts: Molecular Orbitals and Pi Systems
To gain a deeper understanding, visualizing the molecular orbitals (MOs) involved is crucial.
Molecular Orbital Diagrams: Visualizing Electron Distribution
Molecular orbital diagrams depict the constructive and destructive interference of atomic orbitals to form molecular orbitals. In a double bond, the combination of two p orbitals forms two molecular orbitals: a bonding pi (π) orbital (lower in energy) and an antibonding pi* (π*) orbital (higher in energy). The two pi electrons occupy the lower-energy bonding pi orbital.
Delocalization and Resonance Structures: A Simplified View
Resonance structures are a simplified way of representing delocalized pi electrons. They depict the different possible arrangements of pi electrons within a conjugated system. While none of the individual resonance structures accurately represents the molecule, their combination provides a better overall representation of the delocalized electron density.
Conclusion: The Importance of Pi Electrons in Organic Chemistry
Understanding the number of pi electrons in a double bond and how they are distributed within molecules is fundamental to understanding their reactivity, stability, and other chemical properties. The concepts discussed in this article—sigma and pi bonds, aromaticity, conjugated systems, and molecular orbitals—form a cornerstone of organic chemistry. From simple alkenes to complex aromatic compounds and polymers, the distribution and behavior of pi electrons define a molecule's unique characteristics and behavior. A comprehensive understanding of this topic is crucial for success in advanced organic chemistry and related fields.
Latest Posts
Latest Posts
-
An Organism That Eats Only Plants
Apr 02, 2025
-
What Is 1 To The 5th Power
Apr 02, 2025
-
Takes The Place Of A Noun
Apr 02, 2025
-
What Is The Fraction Of 0 04
Apr 02, 2025
-
How Does The Use Of Fertilizer Affect The Nitrogen Cycle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Pi Electrons In A Double Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
