How Many Neutrons Does Barium Have
listenit
Mar 31, 2025 · 5 min read
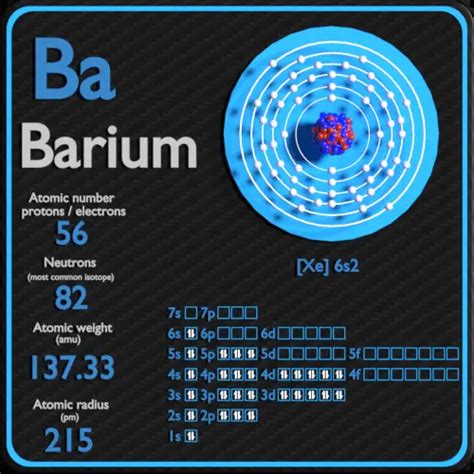
Table of Contents
How Many Neutrons Does Barium Have? Understanding Isotopes and Atomic Structure
Barium, a fascinating alkaline earth metal with the symbol Ba and atomic number 56, presents a unique challenge when it comes to determining the number of neutrons it possesses. Unlike the atomic number, which is fixed and defines the element, the number of neutrons in barium can vary. This variation stems from the existence of isotopes. This article delves into the intricacies of barium's isotopic composition, explaining how to calculate neutron numbers, and exploring the significance of this variation in various applications.
What are Isotopes?
Before we delve into the specifics of barium's neutron count, it's crucial to understand the concept of isotopes. Isotopes are atoms of the same element that share the same number of protons (and thus the same atomic number) but differ in the number of neutrons. This difference in neutron number affects the atomic mass of the isotope. Since the number of protons defines the element, isotopes exhibit similar chemical properties but may have slightly different physical properties due to variations in mass.
Barium's Isotopes: A Spectrum of Neutron Numbers
Barium has a total of 40 known isotopes, ranging from ¹²⁰Ba to ¹⁵¹Ba. These isotopes differ significantly in their stability; some are stable while others are radioactive, decaying through various processes. The number of neutrons in each barium isotope can be calculated by subtracting the atomic number (56) from the mass number (the superscript number in the isotopic notation).
For instance:
-
¹³⁸Ba (the most abundant isotope): This stable isotope has a mass number of 138. Therefore, the number of neutrons is 138 - 56 = 82 neutrons.
-
¹³⁷Ba (a significant radioactive isotope): This isotope has a mass number of 137. Thus, the number of neutrons is 137 - 56 = 81 neutrons.
-
¹³⁵Ba (another important isotope): This isotope's mass number is 135. Consequently, it contains 135 - 56 = 79 neutrons.
Calculating Neutron Number: A Simple Formula
Determining the neutron number for any barium isotope is straightforward. You simply need the mass number (A) and the atomic number (Z). The formula is:
Neutron Number (N) = Mass Number (A) - Atomic Number (Z)
Where:
- A represents the mass number (the total number of protons and neutrons).
- Z represents the atomic number (the number of protons, which is unique to each element).
The Significance of Isotopic Variation in Barium
The varied neutron numbers in barium isotopes have crucial implications in several fields:
-
Nuclear Medicine: ¹³⁷Ba is a significant isotope used in nuclear medicine. Its radioactive decay properties make it suitable for various imaging and therapeutic applications. The specific decay characteristics are directly related to its unique neutron number.
-
Geochronology: Certain barium isotopes are employed in radiometric dating techniques to determine the age of geological formations. The relative abundance of different barium isotopes within a sample can offer insights into its age and geological history. Understanding the precise neutron counts within these isotopes is crucial for these dating methods.
-
Materials Science: The properties of barium-containing materials can vary depending on the isotopic composition of the barium used. For example, the neutron number affects the material's density, thermal conductivity, and other physical properties. This becomes particularly important in designing materials for specific applications.
-
Nuclear Physics Research: Studying the properties of different barium isotopes, particularly the radioactive ones, provides valuable information about nuclear structure and decay processes. These studies help scientists gain a deeper understanding of fundamental nuclear forces.
Abundance of Barium Isotopes and Average Neutron Number
It's important to understand that the number of neutrons in barium isn't a fixed value; it varies depending on the specific isotope. While ¹³⁸Ba is the most abundant isotope (with a natural abundance of approximately 71.7%), other isotopes, such as ¹³⁷Ba, ¹³⁶Ba, and ¹³⁴Ba, also exist naturally in smaller proportions. This means that a sample of natural barium will contain a mixture of isotopes, each with its unique number of neutrons.
To calculate an average number of neutrons in a natural barium sample, you would need to consider the isotopic abundance of each isotope and its corresponding neutron number. This calculation requires weighting each isotope's neutron count by its natural abundance. The precise average neutron number would vary slightly depending on the source and geological origin of the barium sample.
Beyond Barium: The Broader Context of Isotopes
The concept of isotopes isn't unique to barium; it applies to all elements. The variations in neutron numbers affect the properties and applications of elements across the periodic table, with implications ranging from nuclear energy to medical diagnostics. Studying isotopes provides invaluable insights into atomic structure, nuclear processes, and the behavior of matter at the fundamental level.
Conclusion: A Deeper Understanding of Barium's Structure
The question "How many neutrons does barium have?" doesn't have a single answer. The number of neutrons in a barium atom depends entirely on the specific isotope being considered. Understanding the concept of isotopes, the methods for calculating neutron numbers, and the implications of isotopic variations are crucial for appreciating the complexities of barium and its applications in various scientific and technological fields. This exploration highlights the importance of considering the isotopic composition when discussing the properties and uses of any element. From nuclear medicine to geological dating and materials science, the diversity of barium isotopes and their respective neutron numbers plays a critical role in shaping our understanding of the world around us. Further research and explorations into the properties of these isotopes will undoubtedly continue to yield valuable insights and advancements across numerous scientific disciplines.
Latest Posts
Latest Posts
-
How Many Square Yards In A Square Mile
Apr 01, 2025
-
Ca Oh 2 Strong Or Weak
Apr 01, 2025
-
What Are Three Parts Of Atp Molecule
Apr 01, 2025
-
How Many Right Angles Does A Quadrilateral Have
Apr 01, 2025
-
Is Magnesium A Gas Liquid Or Solid
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Barium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
