How Many Electrons In The Third Energy Level
listenit
Mar 22, 2025 · 6 min read
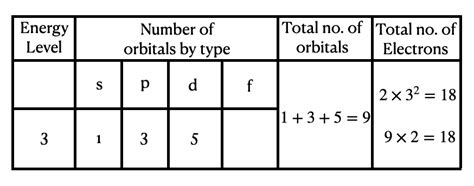
Table of Contents
- How Many Electrons In The Third Energy Level
- Table of Contents
- How Many Electrons in the Third Energy Level? A Deep Dive into Electron Configuration
- Understanding Electron Shells and Subshells
- The Third Energy Level: Unveiling its Electron Capacity
- Electron Filling: The Aufbau Principle and Hund's Rule
- Examples of Electron Configurations and the Third Energy Level
- The Significance of the Third Energy Level in Chemistry
- Beyond the Basics: Exceptions and Refinements
- Conclusion: A Foundation for Further Exploration
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons in the Third Energy Level? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to grasping the behavior of atoms and the properties of elements. A key part of this understanding lies in knowing how many electrons each energy level can hold, particularly the third energy level. This article will delve deep into this topic, exploring the underlying principles of atomic structure, the rules governing electron placement, and the implications of the third energy level's electron capacity.
Understanding Electron Shells and Subshells
Before we tackle the specific question of the third energy level, let's establish a foundation in atomic structure. Electrons, negatively charged particles, orbit the nucleus (containing positively charged protons and neutral neutrons) in specific regions called energy levels or shells. These shells are not physical orbits like planets around a star, but rather represent regions of space where there's a high probability of finding an electron.
Each energy level has a principal quantum number (n), which is a positive integer (1, 2, 3, etc.). The higher the value of 'n', the greater the energy level and the farther it is from the nucleus. The first energy level (n=1) is closest to the nucleus, followed by the second (n=2), third (n=3), and so on.
Crucially, energy levels are further subdivided into subshells, each designated by a letter: s, p, d, and f. These subshells represent different shapes and orientations of electron orbitals within a given energy level.
- s subshell: This subshell has only one orbital and can hold a maximum of 2 electrons.
- p subshell: This subshell has three orbitals, each capable of holding 2 electrons, resulting in a maximum capacity of 6 electrons.
- d subshell: This subshell has five orbitals, accommodating a total of 10 electrons.
- f subshell: This subshell has seven orbitals, holding a maximum of 14 electrons.
The number of subshells present in an energy level is determined by the principal quantum number (n). The first energy level (n=1) has only an s subshell. The second energy level (n=2) has s and p subshells. The third energy level (n=3) has s, p, and d subshells. And so on.
The Third Energy Level: Unveiling its Electron Capacity
Now, let's address the main question: how many electrons can the third energy level (n=3) hold?
The third energy level comprises three subshells: 3s, 3p, and 3d. Let's break down the electron capacity of each:
- 3s subshell: As an s subshell, it has one orbital and can hold a maximum of 2 electrons.
- 3p subshell: As a p subshell, it has three orbitals and can hold a maximum of 6 electrons (3 orbitals x 2 electrons/orbital).
- 3d subshell: As a d subshell, it has five orbitals and can hold a maximum of 10 electrons (5 orbitals x 2 electrons/orbital).
Adding the electron capacities of all three subshells: 2 (3s) + 6 (3p) + 10 (3d) = 18 electrons.
Therefore, the third energy level can hold a maximum of 18 electrons.
Electron Filling: The Aufbau Principle and Hund's Rule
While the third energy level can hold 18 electrons, it doesn't always. The actual number of electrons in the third energy level of an atom depends on the element and its atomic number (the number of protons in the nucleus). Electrons fill energy levels and subshells according to specific rules:
- The Aufbau Principle: Electrons fill the lowest energy levels first. This means that the 1s subshell is filled before the 2s, the 2s before the 2p, and so on. While generally true, there are exceptions, particularly with transition metals.
- Hund's Rule: Within a subshell, electrons fill orbitals individually before pairing up. This minimizes electron-electron repulsion. For example, in a p subshell, each of the three orbitals will receive one electron before any orbital receives a second electron.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, and those two electrons must have opposite spins (represented by ↑ and ↓).
These rules dictate the electron configuration of an atom, which is a notation showing the distribution of electrons among the energy levels and subshells.
Examples of Electron Configurations and the Third Energy Level
Let's look at a few examples to illustrate how the third energy level is filled:
- Sodium (Na, atomic number 11): The electron configuration is 1s²2s²2p⁶3s¹. Sodium has only one electron in its third energy level, residing in the 3s subshell.
- Chlorine (Cl, atomic number 17): The electron configuration is 1s²2s²2p⁶3s²3p⁵. Chlorine has 7 electrons in its third energy level (2 in the 3s and 5 in the 3p).
- Iron (Fe, atomic number 26): The electron configuration is 1s²2s²2p⁶3s²3p⁶4s²3d⁶. Iron has 14 electrons in its third energy level (2 in 3s, 6 in 3p, and 6 in 3d). Note that the 4s subshell fills before the 3d subshell in this case, illustrating an exception to the strict Aufbau principle. This is a common feature of transition metals.
These examples demonstrate how the number of electrons in the third energy level varies depending on the element's atomic number and the rules governing electron filling.
The Significance of the Third Energy Level in Chemistry
The third energy level, and its capacity to hold 18 electrons, plays a crucial role in determining the chemical properties of elements. Elements with partially filled third energy levels are often highly reactive, while those with a completely filled third energy level tend to be less reactive. This reactivity is driven by the tendency of atoms to achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their outermost energy level (valence shell). For many elements, the third energy level constitutes part or all of the valence shell.
The d subshell within the third energy level is particularly important in the chemistry of transition metals. The variable oxidation states and complex formation abilities of these metals are directly related to the electrons in their partially filled 3d orbitals.
Beyond the Basics: Exceptions and Refinements
While the Aufbau principle provides a good framework for understanding electron configuration, it's essential to acknowledge exceptions. The energy levels and subshells are not always perfectly ordered in terms of energy, particularly for heavier atoms. Electron-electron interactions and relativistic effects can cause deviations from the expected filling order. Sophisticated computational methods are sometimes needed to accurately predict electron configurations for more complex atoms.
Conclusion: A Foundation for Further Exploration
Understanding the maximum number of electrons the third energy level can hold—18—is a cornerstone of understanding atomic structure and chemical behavior. The principles of electron filling, governed by the Aufbau principle, Hund's rule, and the Pauli exclusion principle, are essential for predicting and explaining the properties of elements. This knowledge provides a strong foundation for further exploration into the fascinating world of chemistry and the intricacies of atomic structure. Remember to always consult periodic tables and resources which provide detailed electron configurations to fully grasp the complexity and exceptions within electron shell filling.
Latest Posts
Latest Posts
-
In What Organelle Does Photosynthesis Take Place
Mar 23, 2025
-
How Is More Food Increase Carrying Capacity
Mar 23, 2025
-
Length Of A Line Segment Formula
Mar 23, 2025
-
How To Calculate Mass Of Excess Reactant
Mar 23, 2025
-
X 2 Y 2 Z 2
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In The Third Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
