How To Calculate Mass Of Excess Reactant
listenit
Mar 23, 2025 · 6 min read
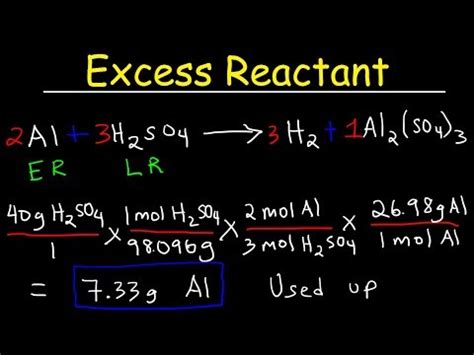
Table of Contents
How to Calculate the Mass of Excess Reactant: A Comprehensive Guide
Determining the mass of excess reactant is a crucial skill in stoichiometry, a cornerstone of chemistry. Understanding this concept allows you to accurately predict the amount of product formed in a chemical reaction and optimize experimental procedures. This comprehensive guide will walk you through the step-by-step process, providing clear explanations and practical examples to solidify your understanding.
Understanding Stoichiometry and Limiting Reactants
Before delving into calculating excess reactant mass, let's refresh our understanding of stoichiometry and limiting reactants. Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction, governed by the balanced chemical equation. The balanced chemical equation provides the molar ratios of reactants and products involved.
A limiting reactant is the reactant that is completely consumed first in a chemical reaction, thereby limiting the amount of product that can be formed. Once the limiting reactant is used up, the reaction stops. The other reactants, present in larger amounts than required, are called excess reactants.
Steps to Calculate the Mass of Excess Reactant
Calculating the mass of the excess reactant involves several key steps:
1. Write and Balance the Chemical Equation:
This is the fundamental first step. Ensure the chemical equation representing the reaction is accurately balanced, meaning the number of atoms of each element is the same on both the reactant and product sides. For example, consider the reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
This equation shows that two moles of hydrogen react with one mole of oxygen to produce two moles of water.
2. Convert the Given Masses to Moles:
Often, you're given the mass (in grams) of each reactant. To perform stoichiometric calculations, you must convert these masses to moles using the molar mass of each substance. The molar mass is the mass of one mole of a substance (in grams/mole) and is found on the periodic table for elements or calculated from the molecular formula for compounds.
Example: Let's say you have 4 grams of hydrogen (H₂) and 32 grams of oxygen (O₂).
- Moles of H₂: Molar mass of H₂ = 2 g/mol. Moles of H₂ = (4 g) / (2 g/mol) = 2 moles
- Moles of O₂: Molar mass of O₂ = 32 g/mol. Moles of O₂ = (32 g) / (32 g/mol) = 1 mole
3. Determine the Limiting Reactant:
Using the balanced chemical equation's molar ratios, determine which reactant is the limiting reactant. Compare the mole ratio of the reactants to the stoichiometric ratio from the balanced equation.
- From the equation: 2 moles of H₂ react with 1 mole of O₂.
- In our example: We have 2 moles of H₂ and 1 mole of O₂. The ratio is 2:1, which matches the stoichiometric ratio. In this specific case, neither reactant is in excess, meaning they react completely. Let's modify the example to illustrate a more typical scenario:
Let's say we have 4 grams of hydrogen (H₂) and 40 grams of oxygen (O₂).
- Moles of H₂: Still 2 moles.
- Moles of O₂: (40 g) / (32 g/mol) = 1.25 moles
Now, comparing the ratio of moles of H₂ to O₂, we have 2:1.25. However, the stoichiometric ratio is 2:1. We have more oxygen than required based on the stoichiometric ratio, indicating that hydrogen is the limiting reactant and oxygen is the excess reactant.
4. Calculate the Moles of Excess Reactant Used:
Using the stoichiometric ratio from the balanced equation and the moles of the limiting reactant, calculate the moles of the excess reactant that reacted.
In our modified example, 2 moles of H₂ react with 1 mole of O₂ (according to the balanced equation). Since we have 2 moles of H₂, this will consume 1 mole of O₂.
5. Calculate the Moles of Excess Reactant Remaining:
Subtract the moles of excess reactant consumed from the initial moles of excess reactant to find the moles of excess reactant remaining.
Initially, we had 1.25 moles of O₂. We consumed 1 mole. Therefore, the moles of O₂ remaining are 1.25 moles - 1 mole = 0.25 moles.
6. Convert Moles of Excess Reactant Remaining to Mass:
Finally, convert the moles of excess reactant remaining back to grams using its molar mass.
Moles of O₂ remaining = 0.25 moles Molar mass of O₂ = 32 g/mol Mass of O₂ remaining = 0.25 moles * 32 g/mol = 8 grams
Therefore, 8 grams of oxygen remain unreacted.
Advanced Scenarios and Considerations
1. Reactions with More Than Two Reactants:
The principles remain the same, but you'll need to compare the mole ratios of all reactants to identify the limiting reactant. A systematic approach, possibly involving several comparisons, is necessary.
2. Reactions with Hydrates or Other Complex Compounds:
Remember to account for the water molecules or other components when calculating molar masses. For example, if dealing with copper(II) sulfate pentahydrate (CuSO₄·5H₂O), include the mass of the five water molecules in the molar mass calculation.
3. Percentage Yield:
In reality, the actual yield of a reaction is often less than the theoretical yield calculated from stoichiometry. The percentage yield accounts for this discrepancy. The mass of excess reactant remaining is still calculated using the theoretical yield.
4. Dealing with Impurities:
If your reactants contain impurities, you need to account for the purity percentage. For example, if you have 90% pure reactant A, you only have 90% of the given mass available for the reaction. You must adjust your mole calculations accordingly.
5. Real-World Applications:
Calculating excess reactant mass has numerous practical applications, including:
- Industrial Chemistry: Optimizing reaction conditions to maximize product yield and minimize waste.
- Pharmaceutical Production: Ensuring the correct stoichiometric ratios for drug synthesis.
- Environmental Science: Modeling chemical reactions in environmental systems.
- Analytical Chemistry: Determining the concentration of unknown substances through titration.
Practice Problems
To solidify your understanding, try these practice problems:
-
Problem 1: 10 grams of calcium carbonate (CaCO₃) reacts with 100 ml of 1M hydrochloric acid (HCl). Calculate the mass of excess reactant remaining after the reaction is complete. The balanced equation is: CaCO₃ + 2HCl → CaCl₂ + H₂O + CO₂
-
Problem 2: 5 grams of sodium hydroxide (NaOH) reacts with 10 grams of sulfuric acid (H₂SO₄). Calculate the mass of excess reactant remaining. The balanced equation is: 2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Remember to follow the six steps outlined above for each problem. Check your answers against the solutions after attempting to solve them independently.
By mastering the calculation of excess reactant mass, you build a strong foundation in stoichiometry and enhance your problem-solving skills in chemistry. The ability to accurately predict and manage reactant quantities is crucial in various scientific disciplines and industrial processes. Remember to practice regularly to improve your proficiency.
Latest Posts
Latest Posts
-
Derivative Of 2 Square Root X
Mar 25, 2025
-
Twice A Number Divided By 6 Is 42
Mar 25, 2025
-
The Sum Of Three Consecutive Integers Is
Mar 25, 2025
-
How Many Centimeters Is In 3 Meters
Mar 25, 2025
-
What Is The Conjugate Acid Of Oh
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Mass Of Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
