How Many Electrons In Carbon Atom
listenit
Mar 28, 2025 · 5 min read
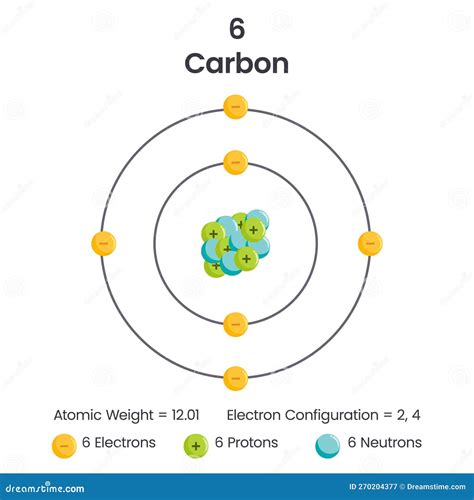
Table of Contents
How Many Electrons in a Carbon Atom? A Deep Dive into Atomic Structure
The seemingly simple question, "How many electrons in a carbon atom?" opens the door to a fascinating exploration of atomic structure, electron configuration, and the fundamental principles of chemistry. Understanding the number of electrons in a carbon atom is crucial for comprehending its unique properties and its role in organic chemistry and countless other fields. This comprehensive guide will not only answer this question definitively but will delve into the broader context of atomic structure, explaining why this number is so significant.
The Fundamental Building Block: The Carbon Atom
Carbon, denoted by the symbol C and atomic number 6, is a nonmetal and the cornerstone of organic chemistry. Its prevalence and versatility stem directly from its electronic structure. The answer to the central question is straightforward: a neutral carbon atom contains six electrons.
Atomic Number and Electrons
The atomic number of an element dictates the number of protons in its nucleus. Since atoms are electrically neutral in their ground state, the number of protons must equal the number of electrons. Therefore, carbon's atomic number of 6 directly translates to six protons and six electrons.
Isotopes and Electron Count
While the number of protons defines an element, the number of neutrons can vary, leading to isotopes. Isotopes of carbon, such as carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C), all have six protons and six electrons. The difference lies in the number of neutrons, impacting the atomic mass but not the electronic structure or the chemical properties in most everyday scenarios. Therefore, regardless of the isotope, the number of electrons remains constant at six.
Electron Shells and Subshells: Understanding Electron Configuration
Electrons don't just orbit the nucleus randomly; they occupy specific energy levels or shells. These shells are further divided into subshells, each capable of holding a specific number of electrons. Understanding this arrangement is key to understanding carbon's reactivity and bonding behavior.
The First Shell (n=1): The 1s Subshell
The closest shell to the nucleus is the first shell (n=1), which can hold a maximum of two electrons. In a carbon atom, this shell is completely filled with two electrons in the 1s subshell. This innermost shell is often referred to as the K shell.
The Second Shell (n=2): The 2s and 2p Subshells
The second shell (n=2) can hold up to eight electrons, distributed among the 2s and 2p subshells. The 2s subshell can hold two electrons, and the 2p subshell can hold six electrons, distributed across three orbitals (2px, 2py, and 2pz).
In a carbon atom, the 2s subshell is filled with two electrons. This leaves four electrons to occupy the 2p subshell. However, only two of the 2p orbitals will be singly occupied, leaving one orbital empty. This configuration is crucial for carbon's ability to form four covalent bonds.
Electron Configuration Notation
The electron configuration of carbon is written as 1s²2s²2p². This notation concisely summarizes the distribution of electrons among the different subshells. The superscript numbers indicate the number of electrons in each subshell.
Carbon's Valence Electrons: The Key to Reactivity
The electrons in the outermost shell, the valence electrons, are responsible for an atom's chemical behavior. In carbon, the outermost shell (n=2) contains four electrons: two in the 2s subshell and two in the 2p subshell. These four valence electrons are the reason carbon can form four covalent bonds, making it the backbone of organic molecules.
Covalent Bonding and Carbon's Versatility
Carbon's four valence electrons allow it to form strong covalent bonds with other atoms, including other carbon atoms. This ability to form long chains, branched structures, and rings is what makes carbon the foundation of organic chemistry and the basis of life as we know it.
The Importance of the 2p Subshell
The partially filled 2p subshell plays a significant role in carbon's ability to form multiple bonds. The unpaired electrons in the 2p orbitals readily participate in covalent bonding, forming single, double, or triple bonds with other atoms. This contributes to the vast structural diversity observed in organic compounds.
Beyond the Neutral Atom: Ions and Charged Species
While a neutral carbon atom has six electrons, it's important to note that carbon can exist in ionized forms. It can lose or gain electrons, resulting in charged species called ions.
Carbon Cations (Positive Ions)
Carbon can lose electrons, becoming a positively charged ion (cation). For example, a carbon cation with a +1 charge (C⁺) would have five electrons. Similarly, a C²⁺ ion would have four electrons. These are typically less stable than the neutral form.
Carbon Anions (Negative Ions)
Less common than cations, carbon can also gain electrons, forming negatively charged ions (anions). A carbon anion with a -1 charge (C⁻) would have seven electrons. A C²⁻ ion would have eight electrons. Again, these are less frequently encountered than the neutral carbon atom.
Conclusion: The Significance of Six Electrons
The seemingly simple answer – six electrons – unlocks a deeper understanding of carbon's fundamental properties and its crucial role in chemistry and biology. The distribution of these six electrons across different energy levels and subshells dictates carbon's bonding capabilities, leading to the incredible diversity of organic molecules and the complexity of life itself. From the simplest hydrocarbons to the most complex biomolecules, carbon's six electrons are the foundation of a vast and intricate world. This knowledge provides a solid base for further exploration into organic chemistry, biochemistry, and materials science. Understanding the electron configuration of carbon is essential for anyone studying the building blocks of the universe and the essence of life itself.
Latest Posts
Latest Posts
-
What Is 6 To The Zeroth Power
Mar 31, 2025
-
Which Intermolecular Force Is The Weakest
Mar 31, 2025
-
How To Calculate Molar Mass Of A Gas
Mar 31, 2025
-
The Weaker The Acid The Stronger The Conjugate Base
Mar 31, 2025
-
How Many Neutrons Does Barium Have
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons In Carbon Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
