The Weaker The Acid The Stronger The Conjugate Base
listenit
Mar 31, 2025 · 6 min read
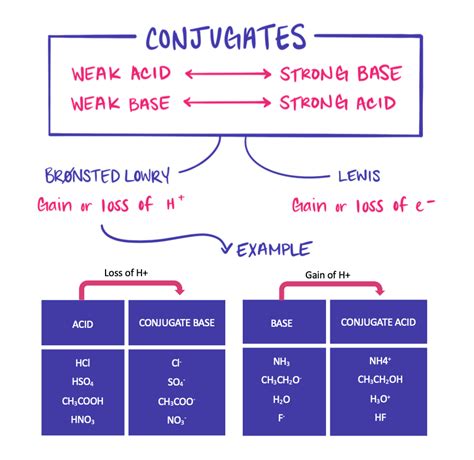
Table of Contents
The Weaker the Acid, the Stronger the Conjugate Base: A Deep Dive into Acid-Base Chemistry
Understanding the relationship between acid strength and the strength of its conjugate base is fundamental to grasping acid-base chemistry. This principle, often summarized as "the weaker the acid, the stronger the conjugate base," governs numerous chemical reactions and phenomena. This article will delve into the intricacies of this concept, exploring its theoretical underpinnings, practical applications, and implications in various chemical systems.
Understanding Acids, Bases, and Conjugate Pairs
Before diving into the central theme, let's establish a firm foundation. Acids are substances that donate protons (H⁺ ions), while bases accept protons. This is according to the Brønsted-Lowry definition of acids and bases. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are known as conjugate acid-base pairs.
For example, consider the reaction between hydrochloric acid (HCl) and water (H₂O):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
In this reaction:
- HCl is the acid, donating a proton.
- H₂O is the base, accepting a proton.
- H₃O⁺ (hydronium ion) is the conjugate acid of H₂O.
- Cl⁻ (chloride ion) is the conjugate base of HCl.
The Strength of Acids and Bases: A Quantitative Measure
The strength of an acid is determined by its tendency to donate a proton. Strong acids, like HCl and HNO₃, readily donate protons, resulting in a high degree of ionization in aqueous solutions. Weak acids, such as acetic acid (CH₃COOH) and formic acid (HCOOH), only partially ionize, meaning they donate protons less readily.
This tendency is quantitatively expressed by the acid dissociation constant (Ka). Ka is the equilibrium constant for the dissociation of an acid in water:
HA + H₂O ⇌ H₃O⁺ + A⁻
Ka = [H₃O⁺][A⁻] / [HA]
A higher Ka value indicates a stronger acid. The pKa, which is the negative logarithm of Ka (pKa = -log Ka), provides a more convenient scale. Lower pKa values correspond to stronger acids.
The Inverse Relationship: Acid Strength and Conjugate Base Strength
The crucial relationship lies in the fact that the strength of an acid and its conjugate base are inversely related. The weaker the acid, the stronger its conjugate base, and vice versa. This is because the equilibrium between the acid and its conjugate base reflects the proton-donating ability.
If an acid is weak (low Ka, high pKa), it means it holds onto its proton tightly. This implies its conjugate base has a strong affinity for protons and readily accepts them, making it a strong conjugate base. Conversely, if an acid is strong (high Ka, low pKa), it readily releases its proton, leaving behind a weak conjugate base that has little affinity for protons.
Explaining the Inverse Relationship: Equilibrium and Le Chatelier's Principle
The inverse relationship can be explained using Le Chatelier's principle, which states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress.
Consider a weak acid, HA, and its conjugate base, A⁻:
HA + H₂O ⇌ H₃O⁺ + A⁻
If we add more of the conjugate base, A⁻, to the equilibrium system, the equilibrium shifts to the left, favoring the formation of the weak acid, HA. This demonstrates that the conjugate base has a strong tendency to accept protons, confirming its strength.
Practical Implications and Applications
The principle "the weaker the acid, the stronger the conjugate base" has far-reaching consequences across various fields:
1. Buffer Solutions:
Buffer solutions are crucial in maintaining a relatively constant pH. They are typically composed of a weak acid and its conjugate base (or a weak base and its conjugate acid). The conjugate base's ability to accept protons helps resist changes in pH when small amounts of acid or base are added.
2. Organic Chemistry:
Understanding conjugate base strength is paramount in organic reactions involving acids and bases. For example, in deprotonation reactions, a strong base is needed to remove a proton from a weak acid. Conversely, a weak base might not be able to deprotonate a stronger acid effectively.
3. Biochemistry:
Many biological systems rely on acid-base equilibria. Amino acids, the building blocks of proteins, contain acidic and basic functional groups. The interplay between acid strength and conjugate base strength plays a crucial role in protein folding, enzyme activity, and other essential biological processes. The effectiveness of various buffers in biological systems depends entirely on this principle.
4. Environmental Chemistry:
Acid rain, resulting from the release of acidic gases into the atmosphere, illustrates the impact of strong acids on the environment. The strong conjugate bases generated can cause significant damage to ecosystems. Understanding the conjugate bases of these acidic pollutants helps in developing mitigation strategies.
Illustrative Examples:
Let's examine a few examples to solidify our understanding:
Example 1: Acetic Acid (CH₃COOH)
Acetic acid is a weak acid (pKa ≈ 4.76). Its conjugate base, acetate (CH₃COO⁻), is therefore a relatively strong conjugate base. Acetate can readily accept a proton, making it an effective base in many reactions.
Example 2: Hydrochloric Acid (HCl)
Hydrochloric acid is a strong acid (pKa ≈ -7). Its conjugate base, chloride (Cl⁻), is an extremely weak conjugate base. Chloride ions have very little tendency to accept protons.
Example 3: Water (H₂O)
Water can act as both an acid and a base (amphoteric). When it acts as an acid, it donates a proton to form hydroxide (OH⁻), which is a strong conjugate base. When it acts as a base, it accepts a proton to form hydronium (H₃O⁺), which is a weak conjugate acid. This highlights that the strength of a conjugate pair depends on the context.
Advanced Concepts and Considerations:
- Solvent Effects: The strength of an acid and its conjugate base can be influenced by the solvent. The polarity and hydrogen-bonding capabilities of the solvent can affect the stability of the acid and its conjugate base, impacting the equilibrium.
- Inductive Effects: In organic acids, the presence of electron-withdrawing or electron-donating groups can significantly influence acid strength and thus the conjugate base's strength. Electron-withdrawing groups increase acid strength, while electron-donating groups decrease it.
- Resonance Effects: Resonance stabilization can affect the stability of both the acid and its conjugate base, influencing their relative strengths. If the conjugate base is resonance-stabilized, the acid is stronger, and the conjugate base is weaker.
Conclusion:
The principle "the weaker the acid, the stronger the conjugate base" is a cornerstone of acid-base chemistry. Understanding this relationship is crucial for predicting reaction outcomes, designing buffer solutions, and interpreting various chemical and biological processes. By exploring the theoretical underpinnings and practical implications of this principle, we gain a deeper appreciation of the intricate balance that governs acid-base equilibria. The concepts discussed here, ranging from equilibrium constants to solvent effects, provide a comprehensive overview of this fundamental concept and its implications across multiple scientific domains. Further exploration of specific examples and advanced concepts will further strengthen your understanding of acid-base chemistry.
Latest Posts
Latest Posts
-
How Much Is 1 4 Pound
Apr 02, 2025
-
What Is The Least Common Multiple Of 9 18
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Weaker The Acid The Stronger The Conjugate Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
