How Many Electrons Does Tin Have
listenit
Mar 24, 2025 · 5 min read
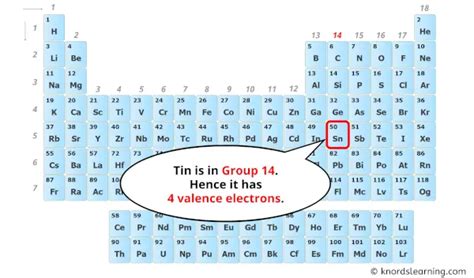
Table of Contents
How Many Electrons Does Tin Have? A Deep Dive into Atomic Structure
Tin, a silvery-white metal known for its malleability and use in various alloys, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is key to comprehending its chemical properties and behaviour. This comprehensive guide will explore not only the simple answer to the question "How many electrons does tin have?" but also delve into the intricacies of its electron configuration, valence electrons, and how this relates to its reactivity and applications.
The Simple Answer: Tin's Electron Count
The atomic number of tin (Sn) is 50. This number, fundamental to the element's identity, directly dictates the number of protons in its nucleus and, crucially for our discussion, the number of electrons orbiting that nucleus in a neutral atom. Therefore, a neutral tin atom possesses 50 electrons.
Delving Deeper: Electron Configuration and Orbitals
While knowing the total electron count is important, understanding where these electrons reside within the atom provides a much richer understanding of tin's characteristics. This involves exploring its electron configuration, which describes the arrangement of electrons in different energy levels and sublevels.
The electron configuration of tin is [Kr] 4d¹⁰ 5s² 5p². Let's break this down:
-
[Kr]: This represents the electron configuration of krypton, a noble gas. It indicates that the inner 36 electrons of tin are arranged identically to those in a krypton atom. These inner electrons are tightly bound to the nucleus and generally don't participate in chemical reactions. They form a stable core.
-
4d¹⁰: This represents ten electrons occupying the 4d sublevel. The 4d sublevel is a higher energy level than the 3d sublevel and has a slightly different shape. These electrons also typically don't participate actively in chemical bonding.
-
5s²: This indicates two electrons in the 5s sublevel. The 5s sublevel is a spherical orbital, and these electrons are relatively close to the outermost shell. They play a significant, although less dominant, role in tin's chemical behavior.
-
5p²: This signifies two electrons in the 5p sublevel. These are valence electrons, the electrons residing in the outermost shell and primarily responsible for tin's chemical reactivity and bonding. These are the electrons most likely to be involved in forming chemical bonds with other atoms.
Understanding Orbitals: Shape and Energy
It's important to visualize the electron arrangement. Each sublevel (s, p, d, f) corresponds to a different type of orbital with a unique shape.
- s orbitals are spherical.
- p orbitals have a dumbbell shape.
- d orbitals are more complex, exhibiting various shapes.
Electrons fill these orbitals according to specific rules, primarily the Aufbau principle (filling lower energy levels first) and Hund's rule (maximizing unpaired electrons before pairing them up). This systematic filling determines the unique electron configuration of each element.
Valence Electrons and Chemical Bonding
The valence electrons, those in the outermost shell (5s² 5p² in tin's case), are the key players in chemical bonding. Tin's four valence electrons allow it to form a variety of compounds through different bonding mechanisms.
Tin commonly exhibits oxidation states of +2 and +4. The +2 oxidation state involves the participation of the two 5p electrons, while the +4 oxidation state involves all four valence electrons (two from 5s and two from 5p). This versatility in oxidation states contributes to tin's diverse range of chemical compounds.
Types of Bonding in Tin Compounds
Tin's chemical behavior is influenced by its ability to participate in various bonding types:
-
Ionic Bonding: Tin can lose its valence electrons to form positively charged ions (Sn²⁺ or Sn⁴⁺), interacting with negatively charged ions (anions) through electrostatic attraction. This is common in compounds with highly electronegative elements like oxygen or chlorine.
-
Covalent Bonding: Tin can also share its valence electrons with other atoms to form covalent bonds, often seen in organotin compounds. These are compounds containing tin-carbon bonds.
-
Metallic Bonding: In pure tin metal, metallic bonding prevails. The valence electrons are delocalized, forming a "sea" of electrons that holds the positively charged tin ions together. This explains tin's metallic properties like conductivity and malleability.
Isotopes and Electron Count
While our discussion has focused on the most common isotope of tin, ¹²⁰Sn, it's important to note that tin has numerous isotopes, each with a different number of neutrons but the same number of protons and electrons in a neutral atom. The variations in neutron count lead to differences in mass, but the electron configuration and chemical properties remain largely consistent across isotopes.
Tin's Applications and its Electron Configuration
The unique electron configuration of tin is directly responsible for its widespread applications in various industries. Its ability to form stable compounds with diverse elements, as well as its metallic properties, makes it a versatile material.
Some key applications include:
-
Alloys: Tin is a crucial component in many alloys, such as bronze (copper and tin) and pewter (tin, copper, antimony, and sometimes bismuth). These alloys benefit from tin's ability to enhance strength, durability, and corrosion resistance.
-
Soldering: Tin-lead solder is widely used in electronics for joining components. The low melting point of tin makes it ideal for this application. Lead-free solders are now increasingly common due to environmental concerns.
-
Coatings: Tin coatings are employed to protect other metals from corrosion. This is particularly valuable in the food packaging industry, where tin cans provide a safe and durable container.
-
Organotin Compounds: These compounds have applications as biocides, catalysts, and stabilizers in plastics. However, due to environmental concerns regarding their toxicity, their usage is becoming more regulated.
-
Other applications: Tin compounds are used in various other applications, including glass manufacturing, ceramics, and pigments.
Conclusion: The Significance of Tin's Electron Count
Understanding the number of electrons in tin – 50 in a neutral atom – is only the first step in comprehending this element's rich chemistry and diverse applications. The detailed electron configuration, emphasizing the role of valence electrons, provides insight into its bonding capabilities and explains its ability to form a vast array of compounds and alloys with unique properties. Tin’s position in the periodic table, along with its electron configuration, directly contributes to its significance in various industrial processes, highlighting the crucial connection between atomic structure and macroscopic properties. From ancient bronze tools to modern electronics, the 50 electrons within each tin atom play a fundamental role in shaping our world.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 12 And 21
Mar 28, 2025
-
What Is 40 Percent Of 28
Mar 28, 2025
-
Do Nonmetals Gain Or Lose Electrons
Mar 28, 2025
-
Five Signs Of A Chemical Reaction
Mar 28, 2025
-
Square Root Of 192 Simplified Radical Form
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Tin Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
