Five Signs Of A Chemical Reaction
listenit
Mar 28, 2025 · 6 min read
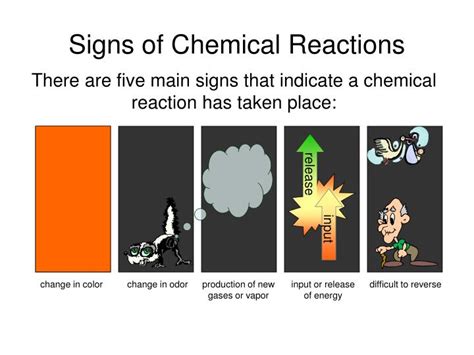
Table of Contents
Five Signs of a Chemical Reaction: A Comprehensive Guide
Chemical reactions are the fundamental processes that govern the transformations of matter around us. From the rusting of iron to the digestion of food, countless chemical reactions shape our world. Recognizing these reactions is crucial in various fields, including chemistry, biology, and engineering. While seemingly complex, identifying a chemical reaction often comes down to observing a few key signs. This comprehensive guide delves into five definitive signs that indicate a chemical reaction is occurring. Understanding these indicators empowers you to better understand the dynamic world of chemistry.
1. A Change in Color: A Visual Cue of Chemical Transformation
One of the most readily observable signs of a chemical reaction is a change in color. This visual cue is often a direct result of the rearrangement of atoms and molecules during the reaction. The change in color signifies the formation of new substances with different electronic structures, which in turn affect how they absorb and reflect light.
Examples of Color Changes Indicating Chemical Reactions:
- Rusting of Iron: The gradual transformation of shiny, grey iron into reddish-brown iron oxide (rust) is a classic example. The color change directly reflects the oxidation reaction where iron reacts with oxygen and water.
- Photosynthesis: While not immediately visually apparent in its entirety, the color change in leaves from green (chlorophyll) to other hues during autumn signifies chemical changes as chlorophyll degrades and other pigments become more visible.
- Iodine Clock Reaction: This popular demonstration in chemistry classrooms vividly shows a dramatic color change. The colorless solution suddenly turns a deep blue-black, demonstrating the rapid formation of a new chemical species.
- Burning of Wood: The change from the brown color of wood to the black ash and the emission of smoke and gases (discussed below) indicate a complex series of chemical reactions.
Why Color Changes Occur: Color is intimately linked to the electronic structure of a substance. When a chemical reaction takes place, bonds are broken and new bonds are formed, leading to a rearrangement of electrons. This rearrangement alters the way the substance interacts with light, resulting in a perceptible color shift. The absorption and reflection of specific wavelengths of light determine the color we see.
2. Formation of a Precipitate: Solid Evidence of a Reaction
A precipitate is a solid that forms from a solution during a chemical reaction. Its appearance is a clear indication that a new substance, insoluble in the solvent, has been created. This formation is often accompanied by a visible clouding or the settling of a solid to the bottom of the container.
Examples of Precipitate Formation:
- Mixing Lead Nitrate and Potassium Iodide: When these two solutions are mixed, a yellow precipitate of lead iodide forms, visibly demonstrating a chemical reaction has taken place.
- Silver Chloride Formation: Mixing silver nitrate and sodium chloride solutions results in the formation of a white, cloudy precipitate of silver chloride. This is a common reaction used in analytical chemistry.
- Formation of Calcium Carbonate: The reaction between calcium ions (present in hard water) and carbonate ions (from bicarbonate or carbon dioxide) can lead to the formation of a calcium carbonate precipitate, commonly seen as limescale.
Understanding Precipitate Formation: A precipitate forms because the product of the chemical reaction is insoluble in the solvent used. The ions involved in the reaction combine to form a stable, solid lattice structure that separates from the solution. The solubility rules of different ionic compounds are crucial in predicting precipitate formation.
3. Evolution of a Gas: Bubbles Indicate a Chemical Change
The release of a gas, often visible as bubbles or effervescence, is a strong indicator of a chemical reaction. The gas produced is a new chemical species formed as a result of the rearrangement of atoms and molecules during the reaction.
Examples of Gas Evolution:
- Reaction of Acids with Metals: Acids, such as hydrochloric acid, react with metals like zinc to produce hydrogen gas, which is observed as bubbles.
- Baking Soda and Vinegar: The classic combination of baking soda (sodium bicarbonate) and vinegar (acetic acid) generates carbon dioxide gas, causing effervescence.
- Decomposition of Hydrogen Peroxide: Hydrogen peroxide decomposes into water and oxygen gas. The oxygen gas can be seen as bubbles, especially when a catalyst like manganese dioxide is added.
- Cellular Respiration: A crucial biological process, cellular respiration releases carbon dioxide gas as a byproduct, which is essential to many cycles in nature.
Identifying Evolved Gases: In many cases, identifying the specific gas produced requires additional testing. However, the observation of gas evolution itself is a definitive sign of a chemical reaction. The nature of the gas can sometimes be inferred based on its smell (caution: never inhale unknown gases!) or other observable characteristics.
4. Temperature Change: Exothermic and Endothermic Reactions
A significant change in temperature during a reaction, either an increase (exothermic) or a decrease (endothermic), is a clear indication of a chemical change. This temperature change reflects the energy changes involved in breaking and forming chemical bonds.
Examples of Exothermic Reactions (release heat):
- Combustion: Burning fuel releases a significant amount of heat, a classic example of an exothermic reaction.
- Neutralization reactions: The reaction between an acid and a base usually produces heat.
- Respiration: The metabolic process of respiration releases heat as a byproduct.
Examples of Endothermic Reactions (absorb heat):
- Dissolving Ammonium Nitrate in Water: This process absorbs heat from the surroundings, resulting in a decrease in temperature.
- Photosynthesis: The process of photosynthesis requires energy from sunlight and is endothermic.
- Melting Ice: Melting ice requires an input of heat.
Understanding Heat Changes: Exothermic reactions release energy in the form of heat because the energy released during bond formation exceeds the energy required to break existing bonds. Conversely, endothermic reactions absorb energy because the energy required to break bonds is greater than the energy released during bond formation.
5. Formation of a New Substance with Different Properties: The Essence of Chemical Change
The ultimate sign of a chemical reaction is the formation of a new substance with properties distinct from the original reactants. This new substance possesses its own unique physical and chemical characteristics, differing from the starting materials.
Examples of New Substance Formation:
- Burning of Methane: Burning methane (natural gas) produces carbon dioxide and water, entirely different substances with different properties.
- Reaction of Sodium and Chlorine: The reaction between sodium metal and chlorine gas forms sodium chloride (table salt), a completely different compound with distinct properties.
- Milk Souring: The souring of milk is caused by the action of bacteria that convert lactose (milk sugar) into lactic acid, a different substance with a sour taste.
Distinguishing Physical and Chemical Changes: It's crucial to distinguish between physical changes (which alter the appearance but not the chemical composition) and chemical changes (which produce new substances). For instance, melting ice is a physical change, while burning wood is a chemical change. The formation of a new substance with different properties is the defining characteristic of a chemical reaction.
Conclusion: Observing Chemical Reactions in Everyday Life
Recognizing the five signs of a chemical reaction – color change, precipitate formation, gas evolution, temperature change, and the formation of a new substance – equips you with a powerful tool to understand the chemical processes occurring around you. From the rusting of a nail to the baking of a cake, countless chemical reactions shape our daily lives. By carefully observing these indicators, you can gain a deeper appreciation for the dynamic world of chemistry. The more you practice observing these key signs, the better you become at identifying and understanding chemical reactions both in a laboratory setting and the world around you. Remember safety precautions when conducting experiments, and always supervise young children who are learning about these concepts.
Latest Posts
Latest Posts
-
6 As A Percentage Of 20
Mar 31, 2025
-
What Is The Name Of The Ionic Compound Cao
Mar 31, 2025
-
Which Function Represents The Given Graph
Mar 31, 2025
-
How To Combine Like Terms With Exponents
Mar 31, 2025
-
Na H2o Naoh H2 Balanced Equation
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Five Signs Of A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
