Na H2o Naoh H2 Balanced Equation
listenit
Mar 31, 2025 · 6 min read
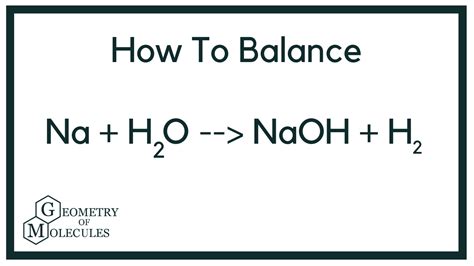
Table of Contents
Understanding the Reaction: Na + H₂O → NaOH + H₂
The reaction between sodium (Na) and water (H₂O) is a highly exothermic single displacement reaction, producing sodium hydroxide (NaOH) and hydrogen gas (H₂). Understanding this reaction requires delving into its balanced equation, the underlying chemistry, safety precautions, and practical applications.
The Balanced Chemical Equation
The unbalanced equation for the reaction is:
Na + H₂O → NaOH + H₂
This equation isn't balanced because the number of atoms of each element isn't equal on both sides. To balance it, we need to ensure the same number of sodium (Na), hydrogen (H), and oxygen (O) atoms are present on both the reactant (left) and product (right) sides.
The balanced equation is:
2Na + 2H₂O → 2NaOH + H₂
This balanced equation shows that two atoms of sodium react with two molecules of water to produce two molecules of sodium hydroxide and one molecule of hydrogen gas. This ensures conservation of mass – no atoms are lost or gained during the reaction.
Understanding the Reaction Mechanism
This reaction proceeds in several steps:
Step 1: Initial Interaction
The highly reactive sodium metal readily donates its lone valence electron to the slightly polar oxygen atom in the water molecule. This electron transfer initiates the reaction.
Step 2: Formation of Sodium Hydroxide
The sodium atom loses an electron, becoming a positively charged sodium ion (Na⁺). The water molecule, having accepted the electron, forms a hydroxide ion (OH⁻). These ions then combine to form sodium hydroxide (NaOH), a strong alkali.
Step 3: Hydrogen Gas Evolution
The remaining hydrogen atoms from the water molecules combine to form hydrogen gas (H₂). This gas is released as bubbles, often vigorously, depending on the reaction conditions.
The Exothermic Nature of the Reaction
This reaction is highly exothermic, meaning it releases a significant amount of heat. This heat is enough to ignite the hydrogen gas produced, often resulting in a visible flame and a characteristic "popping" sound. The heat generated is due to the strong ionic bonds formed in the creation of sodium hydroxide. The energy released is sufficient to break the weaker covalent bonds in the water molecule.
Safety Precautions: Handling Sodium and Water
Sodium reacts violently with water. Extreme caution is necessary when performing this reaction. The following precautions should always be followed:
- Small quantities: Use only small amounts of sodium (a few milligrams) in controlled experiments.
- Appropriate glassware: Use a heat-resistant container to perform the reaction.
- Eye protection: Always wear safety goggles to protect your eyes from splashes and potential explosions.
- Gloves: Wear appropriate gloves to prevent skin contact with sodium and sodium hydroxide.
- Ventilation: Perform the reaction in a well-ventilated area or under a fume hood to prevent inhalation of hydrogen gas.
- Fire extinguisher: Have a fire extinguisher readily available in case of a fire.
- Water disposal: Neutralize any leftover sodium hydroxide with a weak acid, like vinegar, before disposing of it according to local regulations.
Practical Applications
Although the reaction itself might seem to have limited direct applications, the underlying principles and products are crucial in several industrial processes.
1. Sodium Hydroxide Production
Sodium hydroxide (NaOH), also known as caustic soda or lye, is a vital chemical used in numerous industrial processes. The reaction between sodium and water, although not a commercially viable method for large-scale NaOH production, demonstrates the fundamental chemistry behind its formation. Industrial NaOH production typically utilizes other more efficient and cost-effective methods like electrolysis of brine.
2. Hydrogen Gas Production
The hydrogen gas (H₂) produced in the reaction is a clean fuel source with various applications, including fuel cells and ammonia synthesis. However, directly using the hydrogen produced from the sodium-water reaction isn't practical due to the risk of explosion and the cost of sodium. Industrial hydrogen production employs electrolysis of water or steam reforming of natural gas.
3. Demonstrating Reactivity and Exothermic Reactions
This reaction is frequently used in chemistry demonstrations to illustrate the reactivity of alkali metals and the concept of exothermic reactions. It serves as a powerful visual aid for students learning about chemical reactions and energy changes.
Understanding the stoichiometry of the Reaction
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. In the balanced equation:
2Na + 2H₂O → 2NaOH + H₂
The stoichiometric coefficients (the numbers before each chemical formula) indicate the molar ratios of the reactants and products. This means:
- 2 moles of sodium react with 2 moles of water.
- 2 moles of sodium hydroxide are produced.
- 1 mole of hydrogen gas is produced.
These ratios are crucial for calculating the amounts of reactants needed or products expected in a reaction. For instance, if we start with 4 moles of sodium, we would need 4 moles of water, and we'd obtain 4 moles of sodium hydroxide and 2 moles of hydrogen gas.
Factors Affecting the Reaction Rate
Several factors can influence the rate at which this reaction proceeds:
- Temperature: Higher temperatures generally increase the reaction rate, as molecules have more kinetic energy, leading to more frequent and energetic collisions.
- Surface area: Increasing the surface area of the sodium metal (e.g., using smaller pieces) increases the reaction rate by providing more contact points for the water molecules.
- Concentration: While the concentration of water is essentially constant (as it's a solvent), increasing the concentration of sodium (if possible in a controlled environment) would lead to a faster reaction.
- Presence of catalysts: Although no catalysts are typically used in this reaction, the presence of certain substances could potentially alter its rate.
Related Reactions and Concepts
Understanding the Na + H₂O reaction allows for a broader comprehension of similar reactions:
- Reactions of other alkali metals with water: Lithium, potassium, rubidium, and cesium also react vigorously with water, following a similar reaction mechanism but with varying degrees of reactivity. Generally, reactivity increases as you go down Group 1 in the periodic table.
- Redox reactions: This reaction is a redox reaction, where sodium is oxidized (loses electrons) and water is reduced (gains electrons). Understanding redox reactions is fundamental in many chemical processes.
- Acid-base reactions: The formation of sodium hydroxide, a strong base, highlights the concept of acid-base chemistry. Sodium hydroxide readily reacts with acids to form salts and water.
Conclusion
The reaction between sodium and water is a fascinating example of a highly exothermic single displacement reaction. Understanding its balanced equation, mechanism, safety precautions, and applications provides a strong foundation in fundamental chemistry principles. The reaction serves as a valuable tool in education, demonstrating key concepts like stoichiometry, redox reactions, and the reactivity of alkali metals. While the direct applications of the reaction itself might be limited, the principles involved and the products generated are vital across various industrial processes. Remember, safety should always be the paramount concern when handling reactive chemicals like sodium.
Latest Posts
Latest Posts
-
Least Common Multiple Of 12 And 4
Apr 02, 2025
-
What Does 2 1 2 Mean
Apr 02, 2025
-
One Goal Of Nativist Groups In The Late 1800s Was
Apr 02, 2025
-
3 4 3 4 Equals How Many Cups
Apr 02, 2025
-
How Much Is 1 4 Pound
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Na H2o Naoh H2 Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
