Do Nonmetals Gain Or Lose Electrons
listenit
Mar 28, 2025 · 6 min read
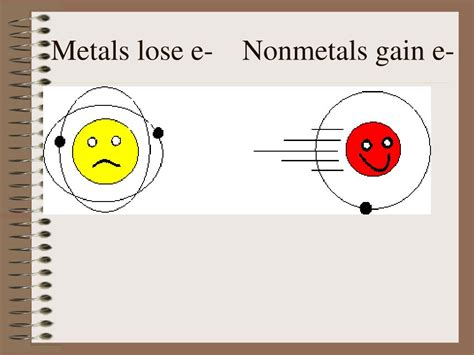
Table of Contents
Do Nonmetals Gain or Lose Electrons? Understanding Electron Behavior in Non-Metallic Elements
The question of whether nonmetals gain or lose electrons is fundamental to understanding their chemical behavior and the formation of chemical bonds. Unlike metals, which readily lose electrons to achieve a stable electron configuration, nonmetals exhibit a contrasting behavior. This article delves into the intricacies of electron behavior in nonmetals, exploring the driving force behind their interactions and the consequences for their chemical properties and reactivity.
The Octet Rule: The Driving Force Behind Electron Gain
The fundamental principle governing the electron behavior of nonmetals is the octet rule. This rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell (valence shell). Exceptions exist, particularly for elements in the first and second periods (hydrogen, helium, lithium, beryllium, boron, etc.), but the octet rule provides a useful framework for understanding the vast majority of chemical interactions.
For nonmetals, achieving a full octet often involves gaining electrons. Their valence shells are typically partially filled, and gaining electrons allows them to reach the stable, lower-energy configuration of a complete octet. This is a thermodynamically favorable process, meaning it releases energy and leads to a more stable system.
Contrasting with Metals: A Tale of Two Behaviors
Metals, on the other hand, typically lose electrons to achieve a stable electron configuration. Their valence electrons are relatively loosely held and readily given up to form positively charged ions (cations). This difference in electron behavior is a key distinction between metals and nonmetals and underpins their different chemical properties and reactivity.
How Nonmetals Gain Electrons: Ionization and Electronegativity
The process by which nonmetals gain electrons is closely related to two key concepts: ionization energy and electronegativity.
Ionization Energy: The Energy Required to Remove an Electron
Ionization energy refers to the energy required to remove an electron from a neutral atom or ion in the gaseous phase. For nonmetals, the ionization energy is relatively high. This high ionization energy reflects the strong attraction between the negatively charged electrons and the positively charged nucleus, making it energetically unfavorable to remove electrons from nonmetal atoms. Therefore, instead of losing electrons, they opt for the energetically favorable process of gaining electrons.
Electronegativity: The Tendency to Attract Electrons
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Nonmetals generally have high electronegativities. This high electronegativity reflects their tendency to pull electrons towards themselves, effectively gaining electrons in the process of forming chemical bonds. The higher the electronegativity, the stronger the pull on electrons, and the more likely a nonmetal is to gain electrons in a chemical interaction.
Consequences of Electron Gain: Anion Formation and Chemical Bonding
When nonmetals gain electrons, they form negatively charged ions called anions. The extra electrons increase the negative charge of the atom, creating an electrostatic imbalance. This anion formation is a crucial step in many chemical reactions and the formation of various compounds.
Ionic Bonding: The Attraction Between Opposite Charges
The formation of anions through electron gain is central to ionic bonding. Ionic bonds form when a metal atom (which tends to lose electrons and form cations) interacts with a nonmetal atom (which tends to gain electrons and form anions). The electrostatic attraction between the positively charged cation and the negatively charged anion holds the atoms together, forming an ionic compound. Examples of ionic compounds include sodium chloride (NaCl), where sodium (Na) loses an electron to chlorine (Cl), and magnesium oxide (MgO), where magnesium (Mg) loses two electrons to oxygen (O).
Covalent Bonding: Sharing Electrons to Achieve Stability
While ionic bonding explicitly involves the transfer of electrons, nonmetals can also achieve a stable octet by sharing electrons through covalent bonding. In covalent bonds, nonmetals share electron pairs to fill their valence shells, effectively completing their octets. This type of bond is prevalent in compounds composed entirely of nonmetals, such as water (H₂O), carbon dioxide (CO₂), and methane (CH₄). Even in covalent bonds, the concept of electronegativity plays a vital role; the electron pair will be more closely associated with the more electronegative atom.
Specific Examples of Nonmetal Electron Gain
Let's examine some specific examples to illustrate the principle of electron gain in nonmetals:
1. Chlorine (Cl): Chlorine, a halogen, has seven valence electrons. To achieve a stable octet, it readily gains one electron to form a chloride ion (Cl⁻). This is why chlorine is highly reactive and readily forms ionic compounds with metals.
2. Oxygen (O): Oxygen has six valence electrons. It typically gains two electrons to form an oxide ion (O²⁻), achieving a stable octet. This strong tendency to gain electrons makes oxygen a highly reactive nonmetal, crucial for numerous biological and chemical processes.
3. Nitrogen (N): Nitrogen has five valence electrons. It commonly gains three electrons to form a nitride ion (N³⁻), although nitrogen also readily forms covalent bonds to complete its octet.
4. Fluorine (F): Fluorine, the most electronegative element, readily gains one electron to form a fluoride ion (F⁻). Its exceptionally high electronegativity makes it extremely reactive and prone to gaining electrons.
Predicting Electron Gain: Periodic Trends and Reactivity
The tendency of nonmetals to gain electrons is predictable based on their position in the periodic table. As you move across a period from left to right, the electronegativity generally increases, leading to an increased tendency to gain electrons. Similarly, as you move up a group, the electronegativity also generally increases, enhancing the likelihood of electron gain.
Exceptions and Limitations of the Octet Rule
While the octet rule provides a valuable framework, it's crucial to acknowledge its exceptions. Some nonmetals, particularly those in the third period and beyond, can accommodate more than eight electrons in their valence shell due to the availability of vacant d-orbitals. This leads to the formation of what are known as expanded octets, observed in compounds like phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
Furthermore, some nonmetals, particularly those in the first and second periods, may exhibit stable configurations with fewer than eight electrons. For example, boron often forms compounds with only six electrons in its valence shell. These exceptions highlight the limitations of strictly adhering to the octet rule, but they do not negate the overall principle that nonmetals tend to gain electrons to achieve a more stable electron configuration.
Conclusion: Electron Gain as a Defining Characteristic of Nonmetals
In conclusion, nonmetals predominantly gain electrons to achieve a stable electron configuration, primarily following the octet rule. This tendency is driven by their high ionization energies and electronegativities, leading to the formation of anions and the establishment of various chemical bonds, both ionic and covalent. Understanding this fundamental aspect of nonmetal behavior is crucial for comprehending their diverse chemical properties, reactivity, and role in the formation of a vast array of compounds. While exceptions exist, the principle of electron gain remains a cornerstone in understanding the chemical world of nonmetals. Their electron-gaining behavior is a defining characteristic that shapes their interactions and their importance in the natural world and various technological applications.
Latest Posts
Latest Posts
-
What Is At The Center Of Every Atom
Mar 31, 2025
-
6 As A Percentage Of 20
Mar 31, 2025
-
What Is The Name Of The Ionic Compound Cao
Mar 31, 2025
-
Which Function Represents The Given Graph
Mar 31, 2025
-
How To Combine Like Terms With Exponents
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Do Nonmetals Gain Or Lose Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
