How Many Electrons Does Mercury Have
listenit
Mar 24, 2025 · 5 min read
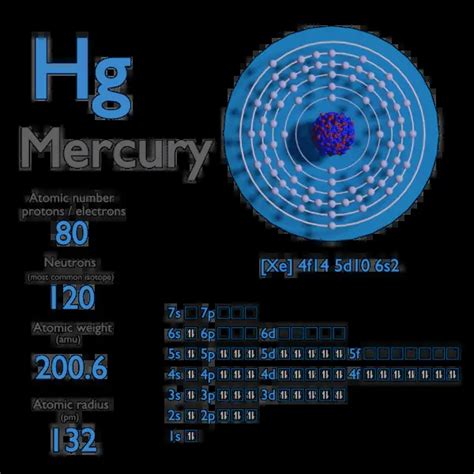
Table of Contents
How Many Electrons Does Mercury Have? Exploring the Atomic Structure of Mercury
Mercury, a fascinating element known for its unique liquid state at room temperature, holds a captivating position in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is crucial to grasping its properties and behavior. This comprehensive guide delves deep into the electronic configuration of mercury, exploring its implications for its chemical reactivity, physical characteristics, and overall significance in science and industry.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we dive into the electron count of mercury, it's essential to understand the fundamental building blocks of an atom:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity.
- Neutrons: Neutral particles (no charge) also found within the nucleus. The number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom. It's the electron configuration that determines an element's chemical properties.
Mercury's Atomic Number and Electron Configuration
Mercury (Hg), element number 80 on the periodic table, possesses 80 protons in its nucleus. In a neutral mercury atom, this means it also has 80 electrons. However, the arrangement of these electrons is what truly dictates its behavior. Electrons occupy specific energy levels or shells, and within these shells, they fill sub-shells according to specific rules governed by quantum mechanics.
The electronic configuration of mercury is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰.
Let's break down this configuration:
- Principal Energy Levels (Shells): The numbers (1, 2, 3, 4, 5, 6) represent the principal energy levels or shells. Electrons closer to the nucleus (lower numbers) have lower energy.
- Subshells: The letters (s, p, d, f) denote subshells within each principal energy level. Each subshell can hold a specific number of electrons: s (2), p (6), d (10), f (14).
- Superscripts: The superscripts (e.g., ² in 1s²) indicate the number of electrons in each subshell.
The Significance of Mercury's Electron Configuration
Mercury's relatively complex electron configuration is responsible for several of its unique properties:
-
Relativistic Effects: Mercury's high atomic number leads to significant relativistic effects on its electrons. This means that the inner electrons are moving at a significant fraction of the speed of light, leading to an increase in their mass and a contraction of their orbitals. This contraction affects the outer electrons and influences mercury's properties, such as its unexpectedly low melting point compared to other transition metals.
-
Chemical Inertness (Relatively): While mercury can form compounds, it's relatively unreactive compared to other metals. This is partly due to the stability of its filled electron shells, particularly the filled 6s subshell. The strong nuclear charge partially shields outer electrons from the nucleus.
-
Liquid State at Room Temperature: The relativistic effects, along with interatomic forces, are key in explaining why mercury is liquid at room temperature. The weak metallic bonding and the influence of relativistic effects on electron orbitals contribute to the low melting point and overall liquid character.
Mercury Isotopes and Electron Count
While the number of electrons in a neutral mercury atom is always 80, different isotopes of mercury exist. Isotopes are atoms of the same element that have different numbers of neutrons. This variation in neutron count alters the atom's mass but does not change the number of protons or electrons in a neutral atom. All isotopes of mercury have 80 electrons in their neutral state.
Common isotopes of mercury include Mercury-196, Mercury-198, Mercury-200, Mercury-201, Mercury-202, and Mercury-204. Each possesses a different number of neutrons, but each neutral atom of these isotopes still has 80 electrons.
Mercury's Role in Science and Industry
Mercury's unique properties and electron configuration have led to its use in various scientific and industrial applications, although many of these applications are being phased out due to its toxicity. Historically, it has been used in:
- Thermometers and Barometers: Mercury's uniform thermal expansion made it ideal for temperature measurement.
- Fluorescent Lamps: Mercury vapor was used in fluorescent lighting due to its emission of ultraviolet light.
- Electrical Switches and Relays: Mercury's conductivity and liquid state facilitated the development of various electrical components.
- Amalgams (Dental Fillings): Mercury's ability to form amalgams with other metals, notably silver, has been utilized in dentistry. However, due to safety concerns, the use of mercury amalgams is declining.
- Catalysis: Mercury compounds have been used as catalysts in certain chemical processes, but due to environmental concerns, safer alternatives are being sought.
Environmental Concerns and Mercury's Toxicity
While mercury has important applications, it's crucial to acknowledge its toxicity and environmental impact. Mercury is a neurotoxin, and exposure can lead to severe health consequences. Its widespread use has led to significant environmental pollution, necessitating careful handling and responsible disposal. The bioaccumulation of mercury in the food chain is a major concern, particularly in aquatic ecosystems.
Conclusion: Understanding Mercury's 80 Electrons
The seemingly simple question, "How many electrons does mercury have?", leads to a deeper exploration of atomic structure, electron configuration, relativistic effects, and the fascinating properties of this unique element. The 80 electrons in a neutral mercury atom are not just a number; they are the key to understanding its liquid state at room temperature, its relatively low reactivity, and its diverse applications, while also highlighting the essential need for careful handling and responsible use given its toxicity. Understanding the electron configuration empowers scientists and engineers to develop safer alternatives and mitigate the environmental impact of this element.
Latest Posts
Latest Posts
-
Where Does Dna Replication Take Place In A Eukaryotic Cell
Mar 26, 2025
-
Salt Dissolves In Water Physical Or Chemical
Mar 26, 2025
-
Polygon With 4 Sides And 4 Angles
Mar 26, 2025
-
What Is The Molecular Shape Of Pf3
Mar 26, 2025
-
How Many Electrons Does Arsenic Have
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Mercury Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
