How Many Electrons Does Arsenic Have
listenit
Mar 26, 2025 · 5 min read
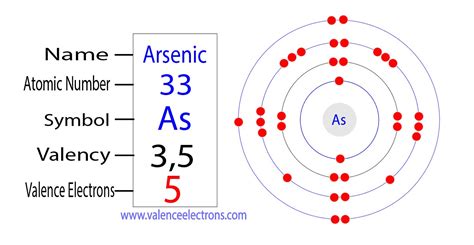
Table of Contents
How Many Electrons Does Arsenic Have? Delving into Atomic Structure and Properties
Arsenic, a metalloid element with fascinating properties and a complex history, holds a unique place in the periodic table. Understanding its fundamental characteristics, particularly its electron configuration, is key to comprehending its behavior and applications. This article will explore the number of electrons in an arsenic atom, delving into the underlying principles of atomic structure and explaining its significance in determining arsenic's chemical reactivity and physical properties.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we delve into the specifics of arsenic, let's briefly review the fundamental components of an atom. Every atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the atomic number of an element and uniquely identifies it on the periodic table.
- Neutrons: Neutrally charged particles also residing in the atom's nucleus. The number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons.
The arrangement of electrons in these shells determines an atom's chemical properties and how it interacts with other atoms. This electron arrangement is described by the element's electron configuration.
Arsenic's Place in the Periodic Table and its Atomic Number
Arsenic (As) is located in Group 15 (also known as the pnictogens) and Period 4 of the periodic table. Its atomic number is 33, which means a neutral arsenic atom possesses 33 protons in its nucleus. Since the number of electrons in a neutral atom equals the number of protons, a neutral arsenic atom has 33 electrons.
Arsenic's Electron Configuration: Unveiling the Shell Structure
The 33 electrons in an arsenic atom are not randomly distributed. They occupy specific energy levels or shells, following the Aufbau principle and Hund's rule. These rules dictate that electrons fill lower energy levels before higher ones and that electrons individually occupy orbitals within a subshell before pairing up.
The electron configuration of arsenic is: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p³. Let's break this down:
- 1s²: Two electrons occupy the first energy level (shell), specifically the 1s subshell.
- 2s²2p⁶: Eight electrons occupy the second energy level, with two in the 2s subshell and six in the 2p subshell.
- 3s²3p⁶: Eight electrons occupy the third energy level, with two in the 3s subshell and six in the 3p subshell.
- 4s²3d¹⁰: Eighteen electrons occupy the fourth energy level. Two are in the 4s subshell, and ten are in the 3d subshell (note that the 3d subshell fills after the 4s subshell).
- 4p³: Three electrons occupy the 4p subshell of the fourth energy level. This partially filled p subshell is crucial in determining arsenic's chemical reactivity.
The Significance of Arsenic's Electron Configuration: Chemical Reactivity and Bonding
The electron configuration directly influences arsenic's chemical behavior. The three electrons in the 4p subshell are valence electrons – the electrons involved in chemical bonding. This means arsenic can readily form covalent bonds by sharing these electrons with other atoms. It can also gain three electrons to achieve a stable octet, forming arsenide anions (As³⁻) in ionic compounds.
Arsenic's ability to form multiple bonds with different elements contributes to its diverse chemistry and the formation of a wide range of compounds. These compounds find applications in various fields, including semiconductors, pesticides, and medicine (though the toxicity of arsenic limits its use in many applications).
Isotopes of Arsenic and their Electron Configurations
While the number of protons defines an element, the number of neutrons can vary, leading to isotopes. Arsenic has several naturally occurring isotopes, the most common being Arsenic-75 (⁷⁵As). While the number of neutrons differs between isotopes, the number of electrons in a neutral atom remains the same—33 for all arsenic isotopes. The mass number (protons + neutrons) changes, but the electron configuration of a neutral atom remains consistent.
Arsenic's Properties: A Consequence of its Electronic Structure
The properties of arsenic, both physical and chemical, are a direct consequence of its electron configuration and atomic structure.
- Metalloid Nature: Arsenic displays properties of both metals and nonmetals. Its partially filled p subshell contributes to its semiconducting behavior.
- Toxicity: Arsenic's toxicity is a well-known phenomenon. The chemical reactivity of arsenic allows it to interact with biological molecules, disrupting cellular processes and leading to poisoning. The mechanisms of arsenic toxicity are complex and involve interactions with various enzymes and cellular components.
- Applications: Despite its toxicity, arsenic finds applications in specific fields. Its semiconducting properties make it useful in electronics, while some arsenic compounds have found use in medicine (though with careful consideration of toxicity).
Beyond the Basics: Advanced Concepts Related to Arsenic's Electrons
A deeper understanding of arsenic's electron behavior involves more advanced concepts:
- Ionization Energies: The energy required to remove an electron from an arsenic atom. These energies increase as more electrons are removed, reflecting the increasing attraction of the nucleus for the remaining electrons.
- Electron Affinity: The energy change when an electron is added to a neutral arsenic atom. Arsenic's electron affinity reflects its tendency to gain electrons to achieve a stable octet.
- Orbital Hybridization: The mixing of atomic orbitals to form hybrid orbitals involved in bonding. This concept helps explain the geometry of arsenic-containing molecules.
- Spectroscopy: Techniques like X-ray photoelectron spectroscopy (XPS) can be used to probe the energy levels and binding energies of electrons in arsenic compounds.
Conclusion: The Crucial Role of Electrons in Arsenic's Chemistry and Applications
The number of electrons in an arsenic atom, its electron configuration, and the behavior of those electrons are fundamental to understanding its properties and its roles in various fields. The 33 electrons are not merely a numerical value; they dictate arsenic's chemical reactivity, its semiconducting behavior, and ultimately its applications and potential toxicity. Understanding the interplay of these electrons, their arrangement within the atom, and the consequences for chemical bonding is crucial for researchers, scientists, and anyone interested in the fascinating world of chemistry and materials science. The detailed exploration of arsenic's electronic structure helps us appreciate the intricate connections between an element's fundamental properties and its macroscopic behavior. From the microcosm of atomic interactions to the macrocosm of its applications, the 33 electrons of arsenic play a defining role in its story.
Latest Posts
Latest Posts
-
What Is The Molecular Geometry Of Ccl4
Mar 26, 2025
-
The Basic Structural Unit Of The Body Is The
Mar 26, 2025
-
How Many Neutrons Does Fe Have
Mar 26, 2025
-
What Is 0 9 As A Percentage
Mar 26, 2025
-
What Percent Of 77 Is 7
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Arsenic Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
