How Many Neutrons Does Fe Have
listenit
Mar 26, 2025 · 5 min read
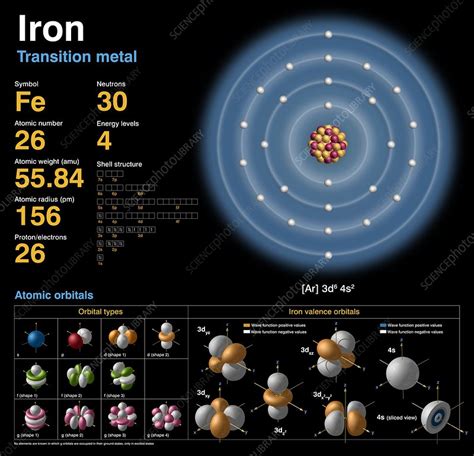
Table of Contents
How Many Neutrons Does Fe Have? Understanding Isotopes and Atomic Structure
Iron (Fe), a ubiquitous element vital for life and industry, presents a fascinating study in atomic structure. A key aspect of understanding iron is determining the number of neutrons it possesses. However, the answer isn't a simple single number. The number of neutrons in an iron atom depends on its isotope. This article delves into the intricacies of iron's isotopes, explaining what isotopes are, how they affect neutron count, and the implications for various applications of iron.
Understanding Isotopes: The Key to Neutron Count Variation
Before we can determine the number of neutrons in iron, we need to grasp the concept of isotopes. Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. The number of protons defines the element; changing the number of protons changes the element entirely. However, the number of neutrons can vary within an element, leading to different isotopes.
This variation in neutron number doesn't alter the chemical properties of the element significantly, as chemical properties are primarily determined by the number of electrons (which equals the number of protons in a neutral atom). However, the different number of neutrons does affect the atom's mass and its nuclear stability, impacting its physical properties and radioactive behavior.
Atomic Structure Basics: Protons, Neutrons, and Electrons
To fully understand isotopes, let's revisit the fundamental components of an atom:
-
Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity. Iron (Fe) has an atomic number of 26, meaning every iron atom has 26 protons.
-
Neutrons: Neutrally charged particles also located in the atom's nucleus. Unlike protons, the number of neutrons can vary within the same element, leading to isotopes. Neutrons contribute significantly to the atom's mass.
-
Electrons: Negatively charged particles that orbit the nucleus. The number of electrons in a neutral atom equals the number of protons. Electrons determine the chemical behavior of an element.
Iron's Isotopes: A Spectrum of Neutron Numbers
Iron, with its atomic number of 26, possesses several naturally occurring isotopes. The most common isotopes are:
-
⁵⁴Fe: This isotope has 26 protons and 28 neutrons (54 - 26 = 28). It accounts for approximately 5.8% of naturally occurring iron.
-
⁵⁶Fe: This is the most abundant isotope of iron, comprising about 91.7% of naturally occurring iron. It has 26 protons and 30 neutrons (56 - 26 = 30).
-
⁵⁷Fe: This isotope has 26 protons and 31 neutrons (57 - 26 = 31). It's present in approximately 2.2% of naturally occurring iron.
-
⁵⁸Fe: This isotope has 26 protons and 32 neutrons (58 - 26 = 32) and comprises about 0.3% of naturally occurring iron.
Less Abundant Iron Isotopes
While less common, several other iron isotopes exist, both naturally occurring in trace amounts and artificially produced. These include radioactive isotopes that decay over time, releasing particles and energy. The number of neutrons in these less abundant isotopes varies, further expanding the range of neutron counts for iron atoms.
Calculating Neutron Number: A Simple Formula
Calculating the number of neutrons in any isotope is straightforward. You simply subtract the atomic number (number of protons) from the mass number (total number of protons and neutrons):
Number of neutrons = Mass number - Atomic number
For example, for the most abundant isotope of iron, ⁵⁶Fe:
Number of neutrons = 56 - 26 = 30
The Significance of Isotope Abundance and Neutron Count
The abundance of different iron isotopes has significant implications in various fields:
-
Geochemistry: Isotopic ratios of iron can provide valuable insights into geological processes and the formation of rocks and minerals. Analyzing the relative abundance of different iron isotopes helps scientists understand past geological events.
-
Cosmochemistry: The abundance of ⁵⁶Fe in the universe is a crucial piece of evidence supporting theories about stellar nucleosynthesis, the process by which elements are created in stars.
-
Materials Science: The properties of iron-based materials, such as steel, can be influenced by the isotopic composition of the iron used. Different isotopes may exhibit slightly different properties, impacting the final product's performance.
-
Medical Applications: ⁵⁹Fe, a radioactive isotope of iron, is used in medical imaging and diagnostics. Its radioactive decay allows doctors to trace the movement of iron in the body, helping to diagnose iron-related disorders such as anemia.
Iron's Role in Biology and its Isotopic Variations
Iron plays a crucial role in biological systems, primarily as a component of hemoglobin, the protein responsible for oxygen transport in blood. The isotopic composition of iron in biological samples can provide information about diet, environmental exposure, and metabolic processes. Scientists utilize stable iron isotopes (like ⁵⁴Fe and ⁵⁶Fe) to study iron uptake and metabolism in living organisms.
Conclusion: A Deeper Understanding of Iron and its Neutrons
In conclusion, the question "How many neutrons does Fe have?" doesn't have a single answer. Iron exists in various isotopic forms, each possessing a different number of neutrons. The most common isotopes, ⁵⁴Fe, ⁵⁶Fe, ⁵⁷Fe, and ⁵⁸Fe, exhibit neutron numbers of 28, 30, 31, and 32 respectively. The relative abundance of these isotopes is significant in various scientific disciplines, providing valuable insights into geological processes, stellar evolution, material science, and biological systems. Understanding iron's isotopic composition is crucial for comprehending its widespread roles in nature and its various applications. The simple formula of subtracting the atomic number from the mass number allows for straightforward calculation of the neutron count in any given iron isotope. Further research into the subtle variations in isotopic abundances continues to unveil the rich complexity of this essential element.
Latest Posts
Latest Posts
-
Is Water Renewable Or Non Renewable Resource
Mar 29, 2025
-
How To Find Va And Ha
Mar 29, 2025
-
4 X 3 R 2x 5 3x 7 9x
Mar 29, 2025
-
Is Ml And Mg The Same
Mar 29, 2025
-
How To Write In Logarithmic Form
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Fe Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
