What Is The Molecular Geometry Of Ccl4
listenit
Mar 26, 2025 · 5 min read
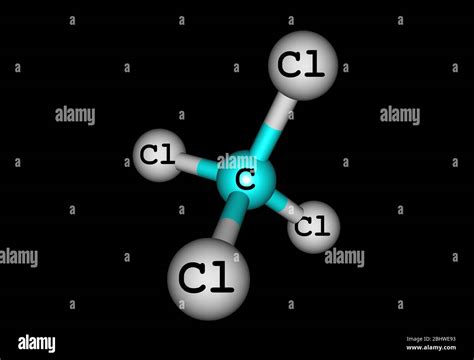
Table of Contents
What is the Molecular Geometry of CCl₄? A Deep Dive into Carbon Tetrachloride's Structure and Properties
Carbon tetrachloride (CCl₄), also known as tetrachloromethane, is a simple yet fascinating molecule with significant implications in various fields, from its past use as a refrigerant and solvent to its current role in research and industrial applications. Understanding its molecular geometry is crucial to comprehending its chemical behavior and properties. This article will delve into the molecular geometry of CCl₄, exploring its bonding, shape, polarity, and other relevant characteristics.
Understanding Molecular Geometry
Molecular geometry, also known as molecular structure, describes the three-dimensional arrangement of atoms within a molecule. It dictates many of the molecule's physical and chemical properties, including its reactivity, polarity, and boiling point. Determining the molecular geometry involves considering several factors:
- Lewis Structure: This represents the arrangement of atoms and valence electrons in a molecule, showing bonding and lone pairs.
- Valence Shell Electron Pair Repulsion (VSEPR) Theory: This theory predicts the geometry by minimizing the repulsion between electron pairs (both bonding and lone pairs) around a central atom.
- Hybridization: This involves the mixing of atomic orbitals to form hybrid orbitals that participate in bonding.
The Lewis Structure of CCl₄
Carbon (C) has four valence electrons, and each chlorine (Cl) atom has seven. To achieve a stable octet, carbon shares one electron with each of the four chlorine atoms, forming four single covalent bonds. This results in the following Lewis structure:
Cl
|
Cl-C-Cl
|
Cl
Each chlorine atom has a complete octet, and the carbon atom also has a complete octet, fulfilling the octet rule.
Applying VSEPR Theory to CCl₄
The VSEPR theory predicts the molecular geometry based on the number of electron pairs around the central atom. In CCl₄, carbon is the central atom surrounded by four bonding pairs and zero lone pairs. According to VSEPR theory, four electron pairs arrange themselves in a tetrahedral geometry to minimize repulsion.
Tetrahedral Geometry Explained
A tetrahedron is a three-dimensional shape with four triangular faces, four vertices, and six edges. In CCl₄, the carbon atom is at the center of the tetrahedron, and the four chlorine atoms occupy the four vertices. The bond angles between any two chlorine atoms are approximately 109.5 degrees.
Hybridization in CCl₄
The carbon atom in CCl₄ undergoes sp³ hybridization. This means that one 2s orbital and three 2p orbitals of carbon combine to form four equivalent sp³ hybrid orbitals. These sp³ hybrid orbitals are directed towards the four corners of a tetrahedron, perfectly aligning with the tetrahedral geometry predicted by VSEPR theory. Each of these sp³ hybrid orbitals overlaps with a 3p orbital of a chlorine atom to form a sigma (σ) bond.
Polarity of CCl₄: A Seemingly Contradictory Property
Although the individual C-Cl bonds are polar (chlorine is more electronegative than carbon), the overall molecule is nonpolar. This is due to the symmetrical tetrahedral arrangement of the chlorine atoms. The individual bond dipoles cancel each other out, resulting in a net dipole moment of zero. This nonpolarity significantly impacts CCl₄'s physical and chemical properties, making it immiscible with water and a good solvent for nonpolar substances.
Physical and Chemical Properties Influenced by Geometry
The tetrahedral geometry and nonpolar nature of CCl₄ significantly influence its properties:
- Boiling Point: Relatively low boiling point compared to similar-sized molecules due to the weak London dispersion forces between nonpolar molecules.
- Solubility: Insoluble in water (a polar solvent) but soluble in nonpolar organic solvents.
- Density: Denser than water.
- Reactivity: Relatively unreactive compared to other chlorinated hydrocarbons. However, under specific conditions, it can undergo reactions such as free radical halogenation.
- Toxicity: Extremely toxic, posing serious health risks through inhalation, ingestion, or skin contact. Its use is now highly restricted due to its harmful environmental impact and its role in ozone depletion.
CCl₄ in Various Applications (Past and Present)
Despite its toxicity, CCl₄ had a wide range of applications in the past:
- Refrigerant: Before the discovery of its harmful effects, it was used as a refrigerant in refrigerators and air conditioners.
- Solvent: It was a common solvent in dry cleaning, degreasing, and extracting processes.
- Fire Extinguisher: Due to its density and ability to smother flames, it was used in fire extinguishers, although its toxicity limits its use today.
Current uses are significantly limited due to its environmental and health hazards. However, it still finds limited applications in niche areas such as research and specialized industrial processes.
Comparing CCl₄ to other Tetrahedral Molecules
Many molecules exhibit tetrahedral geometry. Comparing CCl₄ to other tetrahedral molecules helps illustrate the importance of molecular geometry:
- CH₄ (Methane): Both CCl₄ and CH₄ have tetrahedral geometry, but CH₄ is nonpolar and less reactive than CCl₄ due to the lower electronegativity difference between carbon and hydrogen.
- CF₄ (Tetrafluoromethane): Similar to CCl₄ in geometry, CF₄ is also nonpolar but even less reactive due to the high electronegativity of fluorine.
- SiCl₄ (Silicon Tetrachloride): While having a similar tetrahedral structure, SiCl₄ is more reactive than CCl₄ due to the larger size and lower electronegativity of silicon.
Conclusion: The Significance of Understanding CCl₄'s Geometry
The tetrahedral molecular geometry of CCl₄ is fundamental to understanding its unique physical and chemical properties. From its nonpolar nature influencing its solubility and boiling point to its reactivity and past applications, its structure dictates its behavior. Understanding molecular geometry, through VSEPR theory and hybridization concepts, allows scientists to predict and explain the behavior of molecules, which is essential for developing new materials, designing chemical reactions, and assessing environmental impact. While CCl₄'s toxic nature limits its applications, studying its structure provides a valuable case study for illustrating the crucial role of molecular geometry in determining the properties and behavior of chemical compounds. The lessons learned from CCl₄ highlight the importance of understanding the relationship between structure and function in chemistry and the need to consider the environmental and health implications of chemical substances.
Latest Posts
Latest Posts
-
The Elements In Group 1 Are Called The
Mar 29, 2025
-
How To Solve 1 2 2
Mar 29, 2025
-
What Is 17 20 As A Decimal
Mar 29, 2025
-
What Is The Net Ionic Equation Of 2h So42
Mar 29, 2025
-
Common Factors Of 28 And 42
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Geometry Of Ccl4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
