How Many Electrons Does Iodine Have
listenit
Mar 22, 2025 · 5 min read
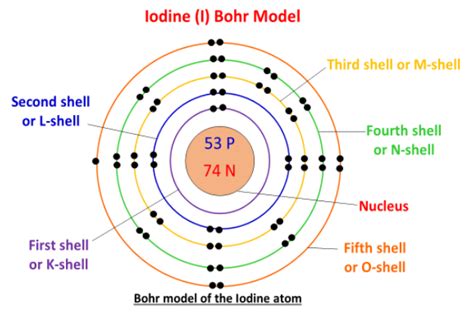
Table of Contents
How Many Electrons Does Iodine Have? A Deep Dive into Atomic Structure
Iodine, a fascinating element with a rich history and crucial role in human biology, sparks curiosity about its atomic makeup. The simple question, "How many electrons does iodine have?" opens the door to a deeper understanding of atomic structure, electron configuration, and the periodic table. This comprehensive article will explore this question, delving into the intricacies of iodine's electronic structure and its implications for its chemical properties and applications.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we determine the number of electrons in iodine, let's establish a foundational understanding of atomic structure. An atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element's atomic number and dictates its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to different isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom, maintaining electrical neutrality.
Iodine's Position on the Periodic Table: Unveiling its Atomic Number
The periodic table is a powerful tool for understanding the properties of elements. Iodine (I), symbolized by the letter 'I', is located in Group 17 (also known as the halogens) and Period 5. Its position provides vital information about its atomic structure. The atomic number of iodine is 53. This means that a neutral iodine atom possesses 53 protons in its nucleus.
The Electron Configuration of Iodine: Orbitals and Shells
Crucially, the atomic number directly dictates the number of electrons in a neutral atom. Since a neutral atom has an equal number of protons and electrons, a neutral iodine atom has 53 electrons.
But knowing the total number of electrons is only half the story. Understanding how these electrons are arranged within the atom is equally important. Electrons occupy specific energy levels or shells, and within those shells, they are further distributed into sub-shells and orbitals. This arrangement, known as the electron configuration, determines the atom's chemical behavior.
The electron configuration of iodine (I) is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁵
Let's break down this configuration:
- 1s²: Two electrons in the first energy level (shell), in the s orbital.
- 2s² 2p⁶: Eight electrons in the second energy level; two in the s orbital and six in the three p orbitals.
- 3s² 3p⁶: Eight electrons in the third energy level; two in the s orbital and six in the three p orbitals.
- 4s² 3d¹⁰ 4p⁶: Eighteen electrons in the fourth energy level; two in the 4s orbital, ten in the five 3d orbitals, and six in the three 4p orbitals.
- 5s² 4d¹⁰ 5p⁵: Seventeen electrons in the fifth energy level; two in the 5s orbital, ten in the five 4d orbitals, and five in the three 5p orbitals.
This electron configuration explains iodine's chemical properties. The five electrons in the 5p subshell are the valence electrons, meaning they are the outermost electrons and participate in chemical bonding. Iodine's tendency to gain one electron to achieve a stable octet (eight electrons in its outermost shell) accounts for its high electronegativity and its formation of -1 anions (I⁻).
Isotopes of Iodine: Variations in Neutron Number
While the number of protons and electrons defines the element, the number of neutrons can vary. These variations create isotopes of an element. Iodine has several isotopes, the most common and stable being iodine-127 (¹²⁷I), which has 53 protons and 74 neutrons. Other isotopes exist but are radioactive and less abundant. It's important to note that the number of electrons remains the same (53) regardless of the isotope, as long as the atom is neutral. The different isotopes vary in their mass number (protons + neutrons) but not their electron count in a neutral state.
The Chemical Behavior of Iodine: Implications of its Electron Configuration
Iodine's 53 electrons, and particularly its five valence electrons, strongly influence its chemical behavior. As a halogen, iodine exhibits several key characteristics:
- High Electronegativity: Iodine has a strong tendency to attract electrons towards itself in a chemical bond. This is a consequence of its relatively high effective nuclear charge and the relatively small size of its valence shell.
- Formation of Iodide Anions: To achieve a stable octet, iodine readily gains one electron to form the iodide anion (I⁻). This anion is involved in numerous chemical compounds.
- Reactivity: Although less reactive than other halogens like fluorine and chlorine, iodine still reacts with many metals and nonmetals to form various iodides.
- Oxidation States: Iodine can exhibit various oxidation states, ranging from -1 (iodide) to +7, showcasing its versatility in chemical reactions.
Iodine's Importance in Biology and Applications: A Multifaceted Element
Iodine's unique electronic structure leads to its importance in various fields:
- Thyroid Hormones: Iodine is essential for the production of thyroid hormones, thyroxine (T₄) and triiodothyronine (T₃). These hormones regulate metabolism, growth, and development. Iodine deficiency can lead to goiter and hypothyroidism.
- Medical Imaging: Radioactive isotopes of iodine, such as iodine-131, are used in medical imaging techniques, such as thyroid scans and treatments for hyperthyroidism and thyroid cancer. The radioactive decay process allows visualization of the thyroid gland.
- Industrial Applications: Iodine and its compounds find various industrial applications, including as catalysts, disinfectants, and in the production of dyes and pharmaceuticals. Its ability to react and bond in diverse ways fuels these applications.
- Photography: Silver iodide (AgI) is used in photographic film as a light-sensitive material. Iodine's reaction with silver contributes to the creation of the photographic image.
Conclusion: The Significance of Iodine's 53 Electrons
The seemingly simple question, "How many electrons does iodine have?", has led us on a journey through the fascinating world of atomic structure and chemical properties. Iodine's 53 electrons, arranged in a specific electron configuration, define its chemical behavior, its biological importance, and its widespread applications. Understanding this electron arrangement is key to appreciating iodine's multifaceted role in science, medicine, and industry. The number 53 is not merely a numerical value; it's a fundamental characteristic that unlocks the understanding of this essential element's properties and significance. This comprehensive exploration highlights the interconnectedness of atomic structure, electron configuration, and the resulting chemical and physical characteristics that make iodine a uniquely vital element.
Latest Posts
Latest Posts
-
Is Corrosion A Physical Or Chemical Property
Mar 23, 2025
-
What Is 0 02 As A Fraction
Mar 23, 2025
-
2 5 As A Percentage And Decimal
Mar 23, 2025
-
In What Organelle Does Photosynthesis Take Place
Mar 23, 2025
-
How Is More Food Increase Carrying Capacity
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Iodine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
