How Many Electrons Can P Orbital Hold
listenit
Mar 28, 2025 · 6 min read
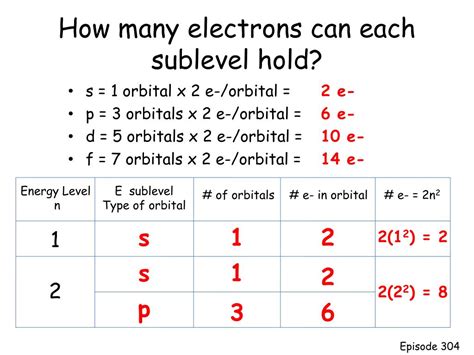
Table of Contents
How Many Electrons Can a p Orbital Hold? A Deep Dive into Atomic Structure
Understanding electron configuration is fundamental to grasping the behavior of atoms and molecules. A crucial part of this understanding lies in knowing how many electrons a specific orbital can hold. This article will delve deep into the specifics of p orbitals, explaining not only their electron capacity but also their shape, energy levels, and significance in chemical bonding.
Understanding Atomic Orbitals: Shells, Subshells, and Orbitals
Before we focus on p orbitals, let's establish a foundational understanding of atomic structure. Electrons within an atom are arranged in energy levels, often visualized as shells surrounding the nucleus. Each shell can hold a specific maximum number of electrons. These shells are further divided into subshells, designated by the letters s, p, d, and f. Each subshell consists of one or more orbitals. An orbital is a region of space where there's a high probability of finding an electron.
Think of it like this: the atom is a house, the shells are floors, the subshells are rooms, and the orbitals are specific areas within those rooms where you're most likely to find someone.
The Significance of Electron Configuration
Understanding electron configuration – the arrangement of electrons within an atom's shells and subshells – is crucial for several reasons:
- Predicting Chemical Properties: The outermost electrons, known as valence electrons, primarily determine an element's reactivity and how it will interact with other atoms.
- Explaining Bonding: The formation of chemical bonds between atoms is directly related to the electron configurations of the participating atoms. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas.
- Understanding Spectroscopy: The absorption and emission of light by atoms are linked to electron transitions between different energy levels and orbitals. This forms the basis of spectroscopic techniques used to identify and analyze substances.
The p Orbital: Shape and Properties
Unlike the s orbital, which is spherical, the p orbital has a dumbbell shape. There are three p orbitals within each p subshell, designated as p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>. These designations refer to the orientation of the orbitals along the x, y, and z axes in three-dimensional space. These orbitals are perpendicular to each other.
Key characteristics of p orbitals:
- Dumbbell Shape: This unique shape influences how p orbitals interact with other orbitals during chemical bonding.
- Three Orbitals per Subshell: The existence of three p orbitals within each p subshell leads to the ability to accommodate more electrons compared to the s subshell.
- Higher Energy than s Orbitals: p orbitals within the same shell possess higher energy than s orbitals. This energy difference impacts the order in which orbitals are filled during electron configuration.
How Many Electrons Can a p Orbital Hold? The Pauli Exclusion Principle
The fundamental principle governing the maximum number of electrons an orbital can hold is the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers describe an electron's state:
- Principal Quantum Number (n): Describes the energy level or shell.
- Azimuthal Quantum Number (l): Describes the subshell (s, p, d, f).
- Magnetic Quantum Number (ml): Describes the specific orbital within a subshell (e.g., p<sub>x</sub>, p<sub>y</sub>, p<sub>z</sub>).
- Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron, either spin up (+1/2) or spin down (-1/2).
Because each electron must have a unique set of quantum numbers, each orbital can hold a maximum of two electrons, one with spin up and the other with spin down. This is true for all types of orbitals, including p orbitals.
The p Subshell and Electron Capacity
Since each p subshell contains three p orbitals (p<sub>x</sub>, p<sub>y</sub>, p<sub>z</sub>), and each orbital can hold two electrons, a p subshell can hold a total of six electrons (3 orbitals x 2 electrons/orbital = 6 electrons).
Filling p Orbitals: Hund's Rule
When filling p orbitals (or any other subshell with multiple orbitals of the same energy), Hund's Rule comes into play. Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons repel each other and prefer to be as far apart as possible. They will first fill each orbital singly, with parallel spins, before pairing up.
P Orbitals and Chemical Bonding
The shape and directional nature of p orbitals play a crucial role in forming chemical bonds, especially covalent bonds. The overlapping of p orbitals from different atoms leads to the formation of pi (π) bonds, which are important in determining the stability and properties of molecules. For example, the double and triple bonds in many organic molecules involve the participation of p orbitals.
Examples of Electron Configurations with P Orbitals
Let's consider some examples to illustrate how p orbitals are filled:
-
Nitrogen (N): Nitrogen has 7 electrons. Its electron configuration is 1s²2s²2p³. This means the 1s and 2s orbitals are filled, and three electrons occupy the three 2p orbitals individually, following Hund's Rule.
-
Oxygen (O): Oxygen has 8 electrons. Its electron configuration is 1s²2s²2p⁴. Here, two of the 2p orbitals are filled, and one orbital contains a single electron.
-
Phosphorus (P): Phosphorus has 15 electrons. Its electron configuration is 1s²2s²2p⁶3s²3p³. This shows the filling of p orbitals across multiple shells.
Advanced Concepts: Hybrid Orbitals and Molecular Geometry
The concept of hybridization expands upon our understanding of atomic orbitals. Hybridization involves the mixing of atomic orbitals (such as s and p orbitals) to form new hybrid orbitals that are better suited for bonding. These hybrid orbitals often have different shapes and energies than the original atomic orbitals. The geometry of molecules can be predicted by considering the hybridization of the central atom and the number of bonding and non-bonding electron pairs.
Conclusion
The p orbital, with its distinct dumbbell shape and capacity to hold up to two electrons, is a fundamental component of atomic structure. Its ability to form multiple bonds and participate in hybridization makes it vital for understanding the vast array of molecules found in nature and created in the laboratory. Understanding the electron capacity of p orbitals, along with the Pauli Exclusion Principle and Hund's Rule, is essential for accurately predicting the behavior of atoms and the formation of chemical bonds. The seemingly simple question of how many electrons a p orbital can hold opens up a world of complex and fascinating concepts within the realm of chemistry.
Latest Posts
Latest Posts
-
What Is 6 To The Zeroth Power
Mar 31, 2025
-
Which Intermolecular Force Is The Weakest
Mar 31, 2025
-
How To Calculate Molar Mass Of A Gas
Mar 31, 2025
-
The Weaker The Acid The Stronger The Conjugate Base
Mar 31, 2025
-
How Many Neutrons Does Barium Have
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can P Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
