How Many Electrons Are Unpaired In The Orbitals Of Nitrogen
listenit
Mar 28, 2025 · 5 min read
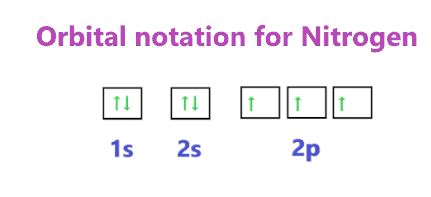
Table of Contents
How Many Unpaired Electrons Are in the Orbitals of Nitrogen? A Deep Dive into Atomic Structure
Nitrogen, a ubiquitous element crucial for life as we know it, presents a fascinating case study in atomic structure and electron configuration. Understanding its electron arrangement reveals key properties and explains its reactivity. This article will delve into the specifics of nitrogen's electron configuration, focusing on the number of unpaired electrons present in its orbitals. We'll explore the underlying principles of atomic orbital theory and the implications of nitrogen's unpaired electrons for its chemical behavior.
Understanding Electron Configuration: The Foundation
Before we tackle nitrogen specifically, let's establish a foundational understanding of electron configuration. Atoms strive for stability, and this is achieved by filling their electron orbitals according to specific rules. These rules govern how electrons are distributed among various energy levels and sublevels within an atom.
The Aufbau Principle and Hund's Rule: Key Principles
The Aufbau principle states that electrons fill the lowest energy orbitals first. Think of it like building a house—you start with the foundation before adding upper floors. Orbitals are filled in order of increasing energy, starting with the 1s orbital, then 2s, 2p, and so on.
Hund's rule adds another layer of complexity. It states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons repel each other, and occupying separate orbitals minimizes this repulsion, leading to a lower energy state. Imagine having separate rooms available; you wouldn't cram into one room if others are empty.
Orbital Notation and Electron Configuration of Nitrogen (N)
Nitrogen (N) has an atomic number of 7, meaning it has 7 protons and 7 electrons in a neutral atom. Following the Aufbau principle and Hund's rule, we can determine its electron configuration:
- 1s² 2s² 2p³
Let's break this down:
-
1s²: The first energy level (n=1) contains one subshell, the 's' subshell, which can hold a maximum of two electrons. Nitrogen fills this subshell completely.
-
2s²: The second energy level (n=2) also contains an 's' subshell, which is again filled with two electrons.
-
2p³: The second energy level also contains three 'p' orbitals (px, py, pz), each capable of holding two electrons. Nitrogen has three electrons remaining, which are distributed individually among these three 2p orbitals according to Hund's rule.
Visualizing Nitrogen's Electron Configuration: Orbital Diagrams
Orbital diagrams provide a visual representation of electron configuration. Each orbital is represented by a box, and electrons are shown as arrows. Arrows pointing up and down represent electrons with opposite spins.
For nitrogen:
- 1s: [↑↓] (two electrons with opposite spins)
- 2s: [↑↓] (two electrons with opposite spins)
- 2p: [↑] [↑] [↑] (three electrons, each occupying a separate 2p orbital)
This clearly shows that nitrogen's three 2p electrons are unpaired.
The Significance of Unpaired Electrons in Nitrogen
The presence of three unpaired electrons in nitrogen's 2p orbitals has profound implications for its chemical behavior and properties.
Reactivity and Bond Formation: The Role of Unpaired Electrons
Unpaired electrons are highly reactive. They readily participate in chemical bonds to achieve a more stable electron configuration. Nitrogen's three unpaired electrons allow it to form up to three covalent bonds, sharing electrons with other atoms. This explains the formation of many nitrogen compounds, including ammonia (NH₃) and nitrogen trichloride (NCl₃).
Paramagnetism: A Consequence of Unpaired Electrons
Substances with unpaired electrons are paramagnetic, meaning they are attracted to magnetic fields. This is because the unpaired electrons' spins create a small magnetic moment. Nitrogen's three unpaired electrons make it a paramagnetic substance. This property can be experimentally verified using a magnetic susceptibility balance.
Delving Deeper: The 2p Orbitals and Their Spatial Orientation
The three 2p orbitals (px, py, pz) are oriented at right angles to each other in three-dimensional space. This spatial orientation plays a role in the geometry of molecules formed by nitrogen. For instance, in ammonia (NH₃), the three hydrogen atoms are arranged in a trigonal pyramidal geometry around the central nitrogen atom, reflecting the spatial arrangement of the nitrogen 2p orbitals.
Comparing Nitrogen's Electron Configuration to Other Elements
By comparing nitrogen's electron configuration to those of other elements, we can gain further insight into the periodic trends in electron arrangement and reactivity.
For example, oxygen (O, atomic number 8) has the electron configuration 1s² 2s² 2p⁴. While oxygen also has electrons in the 2p subshell, it has two unpaired electrons and two paired electrons. This difference in the number of unpaired electrons leads to differences in reactivity and bonding behavior compared to nitrogen. Oxygen is highly reactive and readily forms two covalent bonds, often forming double bonds.
Similarly, comparing nitrogen to other elements in Group 15 (the pnictogens) reveals a pattern of increasing atomic size and decreasing electronegativity as you move down the group. However, the presence of three unpaired electrons in the valence shell remains a common feature, leading to similar bonding patterns across this group.
Conclusion: Nitrogen's Unpaired Electrons – A Key to Its Chemistry
In conclusion, nitrogen possesses three unpaired electrons in its 2p orbitals, as determined by its electron configuration (1s² 2s² 2p³). This fundamental aspect of its atomic structure is crucial in understanding nitrogen's chemical behavior. The unpaired electrons dictate its reactivity, allowing it to form up to three covalent bonds and resulting in the paramagnetic nature of nitrogen. This seemingly simple detail holds the key to the rich and diverse chemistry of nitrogen and its essential role in biological systems and numerous industrial applications. Understanding this core principle strengthens our understanding of chemical bonding, periodic trends, and the properties of matter. Further exploration of nitrogen's chemistry reveals its multifaceted nature and its vital importance in various fields of science and technology.
Latest Posts
Latest Posts
-
How Many Even Numbers Are On A Dice
Mar 31, 2025
-
What Do Plants Have In Common With Animals
Mar 31, 2025
-
What Is 5 16 In Decimal Form
Mar 31, 2025
-
What Is The Square Root Of 70
Mar 31, 2025
-
What Is The Current Model Of The Atom
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are Unpaired In The Orbitals Of Nitrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
