How Many Electrons Are In The Third Energy Level
listenit
Mar 18, 2025 · 5 min read
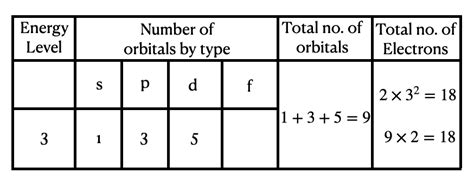
Table of Contents
How Many Electrons Are in the Third Energy Level? A Deep Dive into Atomic Structure
Understanding electron configuration is fundamental to comprehending the behavior of atoms and the properties of matter. A key aspect of this understanding lies in determining the number of electrons that can occupy each energy level within an atom. This article delves deep into the question: How many electrons are in the third energy level? We'll explore the underlying principles of atomic structure, the quantum mechanical model, and the implications of electron configuration for chemical reactivity.
Understanding Energy Levels and Sublevels
Before we tackle the specific question of the third energy level, let's establish a foundational understanding of atomic structure. Electrons don't simply orbit the nucleus like planets around a sun. Instead, they exist in regions of space called electron shells or energy levels. These levels represent different energy states, with electrons in lower energy levels being closer to the nucleus and more tightly bound.
Each energy level is further divided into sublevels, designated as s, p, d, and f. These sublevels have distinct shapes and can hold a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The number of sublevels within an energy level increases as you move further from the nucleus. The first energy level (n=1) only has an s sublevel, the second (n=2) has s and p, and so on.
The Third Energy Level: Unveiling its Electron Capacity
Now, let's focus on the third energy level (n=3). This level contains three sublevels: 3s, 3p, and 3d. To determine the total number of electrons it can hold, we simply add up the maximum number of electrons each sublevel can accommodate:
- 3s sublevel: 2 electrons
- 3p sublevel: 6 electrons
- 3d sublevel: 10 electrons
Total electrons in the third energy level: 2 + 6 + 10 = 18 electrons
Therefore, the third energy level can hold a maximum of 18 electrons.
Electron Configuration and the Aufbau Principle
The way electrons fill energy levels and sublevels follows specific rules. The Aufbau principle states that electrons first fill the lowest energy levels before occupying higher ones. This means that the 1s sublevel is filled before the 2s, and so on. However, the order of filling isn't always strictly sequential due to slight variations in energy levels. The order generally follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
This slightly irregular order is due to the interactions between electrons and the shielding effect of inner electrons.
Illustrative Examples: Electron Configurations of Elements
Let's examine the electron configurations of some elements to illustrate the filling of the third energy level:
- Sodium (Na, atomic number 11): 1s²2s²2p⁶3s¹
- Sodium has one electron in the third energy level (3s¹).
- Chlorine (Cl, atomic number 17): 1s²2s²2p⁶3s²3p⁵
- Chlorine has seven electrons in the third energy level (3s²3p⁵).
- Argon (Ar, atomic number 18): 1s²2s²2p⁶3s²3p⁶
- Argon has eight electrons in the third energy level (3s²3p⁶), completely filling the 3s and 3p sublevels.
- Iron (Fe, atomic number 26): 1s²2s²2p⁶3s²3p⁶4s²3d⁶
- Iron has 14 electrons in the third energy level (3s²3p⁶3d⁶).
These examples demonstrate how the third energy level fills gradually as you progress through the periodic table. Note that the 4s sublevel fills before the 3d sublevel, illustrating the non-strictly sequential nature of the Aufbau principle.
Implications for Chemical Reactivity
The number of electrons in the outermost energy level, known as the valence electrons, determines an element's chemical reactivity. For elements in the third period (row) of the periodic table, the valence electrons reside primarily in the 3s and 3p sublevels. The presence of a full third energy level (18 electrons) results in a stable, unreactive element, such as Argon. Elements with incomplete third energy levels tend to be more reactive, readily gaining or losing electrons to achieve a stable electron configuration, often resembling that of a noble gas.
Beyond the Third Energy Level: Exploring Higher Energy Levels
While this article focuses on the third energy level, it's important to recognize that atoms can have many more energy levels. Higher energy levels contain more sublevels and can hold a significantly larger number of electrons. However, the fundamental principles of electron configuration, the Aufbau principle, and the significance of valence electrons remain consistent regardless of the energy level.
Quantum Mechanical Model and Electron Orbitals
The discussion above relies on the quantum mechanical model of the atom, which provides a more accurate depiction of electron behavior than the older Bohr model. Instead of precisely defined orbits, the quantum mechanical model describes electrons in terms of orbitals. These orbitals represent regions of space where there is a high probability of finding an electron. Each sublevel (s, p, d, f) corresponds to a different type of orbital with a specific shape and orientation.
The Significance of Electron Configuration in Chemistry and Physics
Understanding electron configuration is crucial in various scientific fields:
- Chemistry: It helps predict chemical bonding, reactivity, and the properties of compounds.
- Physics: It's essential for understanding atomic spectra and the interaction of atoms with electromagnetic radiation.
- Materials Science: It plays a critical role in designing and developing new materials with specific properties.
Further Exploration and Conclusion
This detailed exploration has clarified the maximum number of electrons that can occupy the third energy level: 18 electrons. This understanding forms a cornerstone of atomic theory and is essential for comprehending chemical behavior and the properties of matter. Further exploration into quantum mechanics, advanced electron configurations, and the periodic trends in electron arrangements can enhance your understanding even further. Remember that the complexities of atomic structure are fascinating and provide a foundation for countless scientific advancements. By grasping the principles presented here, you can move towards a deeper appreciation for the intricate world of atoms and their electrons.
Latest Posts
Latest Posts
-
What Is The Electron Configuration For Argon
Mar 19, 2025
-
Where Are Metals On The Periodic Table Located
Mar 19, 2025
-
The Most Common Element In The Sun Is
Mar 19, 2025
-
Which Quadrilateral Is Not A Parallelogram
Mar 19, 2025
-
24 Is What Percent Of 30
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In The Third Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
