Where Are Metals On The Periodic Table Located
listenit
Mar 19, 2025 · 5 min read
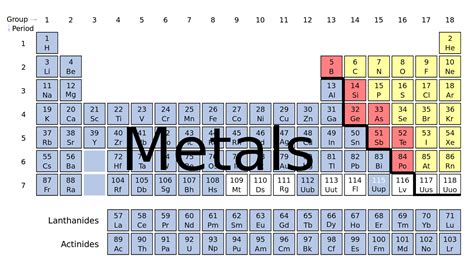
Table of Contents
Where Are Metals Located on the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One of the most fundamental classifications of elements is the division into metals, nonmetals, and metalloids. Understanding where metals reside on the periodic table is crucial to grasping their chemical behavior and applications. This comprehensive guide will delve into the location of metals, exploring their properties and the exceptions that sometimes blur the lines.
The Broad Sweep: Metals Dominate the Left
The most straightforward answer to the question "Where are metals on the periodic table?" is: predominantly on the left and in the center. A vast majority of elements categorized as metals are found to the left of the staircase-like line that separates metals from nonmetals. This line, often jagged, begins near Boron (B) and descends diagonally to include elements like Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), and Polonium (Po). Elements to the left of this line are generally considered metals, while those to the right are nonmetals.
Key Regions of Metallic Abundance:
-
Alkali Metals (Group 1): These highly reactive metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are located in the far left column. Their reactivity stems from their tendency to readily lose one electron to achieve a stable electron configuration.
-
Alkaline Earth Metals (Group 2): Situated next to the alkali metals, this group comprises beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). They are less reactive than alkali metals but still exhibit metallic characteristics like good conductivity and malleability.
-
Transition Metals (Groups 3-12): This large block in the center of the periodic table houses a diverse range of metals. They are known for their variable oxidation states, forming a wide array of colorful compounds. Iron (Fe), copper (Cu), gold (Au), and platinum (Pt) are prime examples of their importance in industry and technology.
-
Inner Transition Metals (Lanthanides and Actinides): These two rows, usually placed separately at the bottom of the table, represent the f-block elements. Lanthanides, also known as rare earth elements, are crucial in various technologies, from magnets to lighting. Actinides, including uranium (U) and plutonium (Pu), are primarily known for their radioactivity.
-
Post-Transition Metals: Located to the right of the transition metals and above the metalloids, these elements exhibit a blend of metallic and nonmetallic properties. They include aluminum (Al), tin (Sn), lead (Pb), and bismuth (Bi). Their properties are less pronounced than the metals in other groups.
The Fuzzy Boundaries: Metalloids and the Staircase
The line separating metals and nonmetals isn't a sharp division. The elements bordering this line, known as metalloids, possess intermediate properties. They exhibit some characteristics of both metals and nonmetals, making their classification less straightforward. Their location on the periodic table highlights this transitional nature. Metalloids are often semiconductors, meaning their conductivity lies between that of metals (good conductors) and nonmetals (insulators). This property makes them essential in electronics.
Understanding the Ambiguity:
The properties of metalloids are highly dependent on factors like temperature and pressure. For instance, silicon (Si) behaves more like a nonmetal at low temperatures but shows increased metallic behavior at higher temperatures. This ambiguity makes placing them definitively in either category challenging. Consequently, their positioning on the periodic table reflects this inherent uncertainty.
Exceptions and Nuances: Not Always Clear-Cut
While the general rule places metals on the left and center of the periodic table, some exceptions exist. Several elements might display properties that don't strictly conform to the typical metallic profile.
Hydrogen: A Unique Case
Hydrogen (H), located in the top left corner, is often placed with the alkali metals due to its single valence electron. However, its properties differ significantly. Under standard conditions, hydrogen behaves as a nonmetal gas. Only under extreme pressure does it exhibit metallic characteristics. This illustrates the complexities of classification and the limitations of a simplistic left-side/right-side division.
Other Notable Exceptions:
Some elements near the metalloid boundary might exhibit some metallic properties depending on specific conditions. The exact behavior depends on their chemical environment, temperature, and pressure. It’s important to understand that the periodic table is a model and not a perfect representation of the complex behavior of individual elements.
Properties of Metals: A Recap
To better understand the location of metals, it's important to review their defining characteristics. These properties, closely linked to their atomic structure, account for their placement on the periodic table.
-
Conductivity: Metals are excellent conductors of electricity and heat due to the mobility of their valence electrons. This property is fundamental to their many technological applications.
-
Malleability and Ductility: Metals can be easily shaped (malleable) and drawn into wires (ductile). This characteristic is directly related to the arrangement of metal atoms and the ease with which they can slide past one another.
-
Luster: Metals generally possess a shiny appearance, a characteristic often referred to as metallic luster. This stems from the interaction of light with their free electrons.
-
Density: Most metals have relatively high densities. Exceptions exist, but generally, metals are considerably denser than nonmetals.
-
Melting and Boiling Points: Metals typically have high melting and boiling points, reflecting the strong metallic bonds between their atoms.
Practical Applications Based on Location:
The location of metals on the periodic table directly relates to their use in various fields. For example:
- Alkali metals: Limited use due to extreme reactivity but crucial in specialized applications.
- Alkaline Earth metals: Magnesium alloys in aerospace, calcium in construction materials.
- Transition metals: Essential in construction, electronics, and catalysis (Iron, Copper, Platinum).
- Post-transition metals: Aluminum in packaging and aerospace, tin in coatings, lead (with decreasing use due to toxicity) in batteries.
Conclusion: A Dynamic Classification
The periodic table provides a powerful framework for understanding the properties and relationships between elements. While the location of metals is generally on the left and center, the line between metals, metalloids, and nonmetals remains somewhat blurred. Understanding this nuance, including the exceptions and the underlying properties of metals, enhances our appreciation of this fundamental tool in chemistry. The periodic table is not a static document; our understanding of elements constantly evolves, leading to refinements in our classification system. However, the broad trend of metals dominating the left side remains a valuable and enduring principle.
Latest Posts
Latest Posts
-
38 Out Of 60 As A Percentage
May 09, 2025
-
How Many Ounces Are In 1 05 Qt
May 09, 2025
-
How To Determine Most Stable Chair Conformation
May 09, 2025
-
A Pair Of Pants With A Marked Price Of 35 00
May 09, 2025
-
18 Ones 9 Tens 2 Hundreds
May 09, 2025
Related Post
Thank you for visiting our website which covers about Where Are Metals On The Periodic Table Located . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.