How Many Electrons Are In Iron
listenit
Mar 14, 2025 · 5 min read
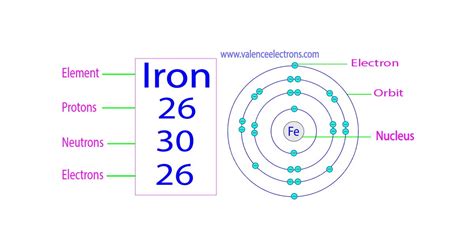
Table of Contents
- How Many Electrons Are In Iron
- Table of Contents
- How Many Electrons Are in Iron? A Deep Dive into Atomic Structure
- Understanding Atomic Structure: Protons, Neutrons, and Electrons
- Iron's Electron Configuration: A Detailed Look
- The Significance of the 3d Orbitals
- Isotopes of Iron and Electron Count
- Iron's Role in Biology and Industry
- Biological Importance:
- Industrial Applications:
- Ions and Electron Count: A Note on Charged Atoms
- Conclusion: Beyond the Simple Answer
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons Are in Iron? A Deep Dive into Atomic Structure
Iron, a ubiquitous element essential to life and industry, possesses a fascinating atomic structure. Understanding its electron configuration is key to grasping its chemical properties and its role in various processes. So, how many electrons does iron have? The simple answer is: 26. But this seemingly straightforward answer opens the door to a much richer exploration of atomic physics and chemistry.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of iron's electrons, let's establish a foundational understanding of atomic structure. An atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; iron, with 26 protons, is unique and distinct from all other elements.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within the same element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons.
It's the electrons, particularly their arrangement and interactions, that determine an atom's chemical behavior.
Iron's Electron Configuration: A Detailed Look
Iron (Fe), with its atomic number of 26, possesses 26 electrons in a neutral atom. These electrons are not randomly distributed; they occupy specific energy levels and sublevels according to the Aufbau principle and Hund's rule. This arrangement dictates its reactivity and magnetic properties.
The electron configuration of iron is written as: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Let's break this down:
- 1s²: The first energy level (n=1) contains one subshell (s), holding a maximum of two electrons.
- 2s² 2p⁶: The second energy level (n=2) contains two subshells: s (holding two electrons) and p (holding six electrons).
- 3s² 3p⁶: The third energy level (n=3) also has s and p subshells, each filled to their maximum capacity.
- 4s² 3d⁶: This is where things get interesting. The fourth energy level (n=4) starts filling before the 3d subshell is completely filled. This is because the 4s subshell has slightly lower energy than the 3d subshell. The 3d subshell can hold a maximum of ten electrons.
This specific arrangement of electrons is responsible for many of iron's unique characteristics.
The Significance of the 3d Orbitals
The partially filled 3d subshell is critical to understanding iron's properties. The presence of unpaired electrons in the 3d orbitals leads to:
- Ferromagnetism: Iron's ability to be strongly attracted to magnets and retain its magnetism is a direct consequence of the unpaired electrons in its 3d orbitals. These electrons interact, creating a cooperative magnetic effect.
- Variable Oxidation States: Iron can exhibit various oxidation states, meaning it can lose different numbers of electrons to form ions. Common oxidation states include +2 (ferrous) and +3 (ferric). The flexibility in electron loss stems from the relatively easy removal of electrons from the 4s and 3d orbitals.
- Complex Formation: Iron's partially filled d-orbitals allow it to form complexes with other molecules or ions, playing crucial roles in biological systems and chemical catalysis. Hemoglobin, the oxygen-carrying protein in blood, relies on iron's ability to form complexes.
Isotopes of Iron and Electron Count
While a neutral iron atom always has 26 electrons, the number of neutrons in the nucleus can vary, resulting in different isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotopes of iron are:
- ⁵⁴Fe: This isotope has 28 neutrons. The electron count remains 26.
- ⁵⁶Fe: This is the most abundant isotope, with 30 neutrons. Again, the electron count remains 26 in a neutral atom.
- ⁵⁷Fe: This isotope has 31 neutrons. Its electron count, in a neutral state, is still 26.
The number of neutrons affects the isotope's mass but does not alter the electron count in a neutral atom. The number of electrons is solely determined by the number of protons.
Iron's Role in Biology and Industry
Iron's unique electronic structure contributes to its crucial roles in various biological and industrial processes.
Biological Importance:
- Oxygen Transport: Hemoglobin, the protein responsible for oxygen transport in blood, contains iron ions that bind to oxygen molecules.
- Enzyme Function: Iron is a cofactor in numerous enzymes, participating in critical metabolic pathways. Cytochromes, involved in electron transport chains, rely on iron.
- Nitrogen Fixation: Certain nitrogen-fixing bacteria utilize iron-containing enzymes to convert atmospheric nitrogen into forms usable by plants.
Industrial Applications:
- Steel Production: Iron is the primary component of steel, a crucial material in construction, manufacturing, and transportation.
- Catalysis: Iron compounds are used as catalysts in various chemical processes, including the production of ammonia.
- Pigments: Iron oxides are used as pigments in paints, cosmetics, and other products.
Ions and Electron Count: A Note on Charged Atoms
It's crucial to remember that the electron count of 26 applies only to neutral iron atoms. When iron loses electrons to form positive ions (cations), the electron count decreases. For example:
- Fe²⁺ (ferrous ion): This ion has lost two electrons, leaving it with 24 electrons.
- Fe³⁺ (ferric ion): This ion has lost three electrons, leaving it with 23 electrons.
Conversely, if iron gains electrons (highly unlikely), it would form a negative ion (anion) with a correspondingly higher electron count.
Conclusion: Beyond the Simple Answer
The simple answer to "How many electrons are in iron?" is 26. However, delving deeper reveals the intricacies of atomic structure and the crucial role electron configuration plays in determining an element's properties. Iron's 26 electrons, specifically their arrangement in the 3d subshell, are responsible for its unique magnetic properties, variable oxidation states, and its indispensable roles in biology and industry. Understanding this fundamental aspect of iron's atomic structure is essential to appreciating its significance in the world around us. The exploration goes beyond a simple number and into the fascinating realm of quantum mechanics and chemical bonding. This provides a foundation for further investigations into the behavior and applications of this vital element.
Latest Posts
Latest Posts
-
How Many Inches Is 12 Yards
Mar 15, 2025
-
Columns On The Periodic Table Are Known As
Mar 15, 2025
-
What The Square Root Of 24
Mar 15, 2025
-
What Percent Is Equivalent To 3 50
Mar 15, 2025
-
Molar Mass Of Al No3 3
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
