Columns On The Periodic Table Are Known As
listenit
Mar 15, 2025 · 7 min read
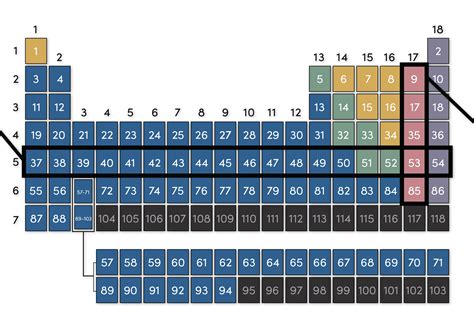
Table of Contents
Columns on the Periodic Table are Known as Groups: A Deep Dive into Chemical Families
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While rows are known as periods, the columns are known as groups or families. Understanding groups is crucial to predicting an element's behavior and its reactions with other elements. This comprehensive article will delve deep into the fascinating world of groups on the periodic table, exploring their characteristics, trends, and significance in various fields.
What Defines a Group?
Elements within the same group share a common characteristic: they possess the same number of valence electrons. Valence electrons are the electrons in the outermost shell of an atom, and they are the key players in chemical bonding. Since elements in a group have the same number of valence electrons, they tend to exhibit similar chemical properties and react similarly with other elements. This similarity is what makes grouping elements so powerful in predicting chemical behavior.
The Significance of Valence Electrons
The number of valence electrons dictates how an element will interact with other elements. Elements strive to achieve a stable electron configuration, often resembling that of a noble gas (Group 18). This drive to achieve stability is the driving force behind chemical reactions. Elements will either gain, lose, or share electrons to reach this stable state. Elements within the same group have similar strategies for achieving this stable configuration, leading to their similar chemical properties.
The Major Groups of the Periodic Table: A Detailed Exploration
The periodic table is broadly categorized into several major groups, each with its unique set of properties:
Group 1: Alkali Metals
Alkali metals are highly reactive metals located in the first column of the periodic table. They all have one valence electron, which they readily lose to form a +1 cation. This makes them highly reactive, especially with water and halogens. Their reactivity increases as you move down the group.
- Key Properties: Soft, silvery-white metals, low melting points, excellent conductors of heat and electricity.
- Examples: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
- Reactions: Vigorously react with water to produce hydrogen gas and a metal hydroxide. React explosively with halogens to form salts.
Group 2: Alkaline Earth Metals
Alkaline earth metals are located in the second column and possess two valence electrons. They are also reactive metals, but less so than alkali metals. They tend to lose both valence electrons to form +2 cations.
- Key Properties: Silvery-white metals, higher melting points than alkali metals, good conductors of heat and electricity.
- Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
- Reactions: React with water, although less vigorously than alkali metals. React with halogens to form salts. Magnesium is notably used in many alloys due to its lightweight and strength.
Group 13: Boron Group
The boron group elements have three valence electrons. This group showcases a transition in properties from metalloids to metals as you move down the group.
- Key Properties: Varying properties, from metalloid (boron) to metals (aluminum, gallium, indium, thallium). Aluminum is lightweight and strong, making it valuable in various applications.
- Examples: Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl).
- Reactions: Boron is relatively unreactive, while the rest show increasing reactivity down the group. Aluminum forms a protective oxide layer, making it resistant to corrosion.
Group 14: Carbon Group
The carbon group elements have four valence electrons. This group is remarkable for its diversity, ranging from nonmetals to metalloids to metals.
- Key Properties: Wide range of properties, from nonmetal (carbon) to metalloids (silicon, germanium) to metals (tin, lead). Carbon forms the basis of organic chemistry and life itself. Silicon is a crucial component in semiconductors.
- Examples: Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb).
- Reactions: Carbon exhibits different bonding properties, forming covalent bonds in organic compounds and network covalent structures in allotropes like diamond and graphite. Silicon and germanium are important semiconductors.
Group 15: Pnictogens
Pnictogens have five valence electrons. This group also demonstrates a variety of properties, with nonmetals at the top and metals at the bottom.
- Key Properties: Diverse properties, ranging from nonmetals (nitrogen, phosphorus) to metalloids (arsenic, antimony) to metal (bismuth). Nitrogen is crucial for life, while phosphorus is a vital component of DNA and RNA.
- Examples: Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), Bismuth (Bi).
- Reactions: Nitrogen is relatively unreactive, while phosphorus exists in several allotropic forms with different reactivities. Arsenic, antimony, and bismuth show metallic properties.
Group 16: Chalcogens
Chalcogens have six valence electrons. This group includes nonmetals, metalloids, and metals, showcasing a range of properties and reactivities.
- Key Properties: Diverse properties, including nonmetals (oxygen, sulfur, selenium) and metalloids (tellurium) and metals (polonium). Oxygen is essential for respiration, while sulfur is used in various industrial applications.
- Examples: Oxygen (O), Sulfur (S), Selenium (Se), Tellurium (Te), Polonium (Po).
- Reactions: Oxygen is highly reactive, supporting combustion. Sulfur forms various compounds, exhibiting multiple oxidation states.
Group 17: Halogens
Halogens possess seven valence electrons. They are highly reactive nonmetals, readily gaining one electron to form a -1 anion. Their reactivity decreases as you move down the group.
- Key Properties: Highly reactive nonmetals, diatomic molecules, various colors and states at room temperature.
- Examples: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
- Reactions: React vigorously with metals to form salts. Fluorine is the most reactive halogen. Chlorine is used in water purification and other industrial processes.
Group 18: Noble Gases
Noble gases have eight valence electrons (except helium, which has two), giving them a stable electron configuration. They are extremely unreactive and are often called inert gases.
- Key Properties: Inert gases, colorless, odorless, monatomic gases. Exist as single atoms, not molecules.
- Examples: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
- Reactions: Historically considered inert, but some heavier noble gases have been shown to form compounds under specific conditions. Helium is used in balloons and cryogenics. Neon is used in lighting.
Transition Metals: A Separate Category
Transition metals occupy the central block of the periodic table. Unlike the main group elements, the transition metals do not follow the straightforward valence electron pattern. They have variable oxidation states and often form complex ions. Their properties are less predictable than those of the main group elements.
- Key Properties: High melting and boiling points, good conductors of heat and electricity, often form colored compounds, exhibit catalytic activity.
- Examples: Iron (Fe), Copper (Cu), Gold (Au), Platinum (Pt), and many others.
- Reactions: Exhibit diverse reactivity due to their variable oxidation states. Many transition metals are essential for biological processes.
Lanthanides and Actinides: The Inner Transition Metals
Lanthanides and actinides are placed separately at the bottom of the periodic table. They are also transition metals but are grouped separately due to their similar chemical properties. They fill the f-sublevel of their electron configurations.
- Key Properties: Similar chemical properties within each series, many are radioactive (actinides).
- Examples: Lanthanum (La) to Lutetium (Lu) for Lanthanides, Actinium (Ac) to Lawrencium (Lr) for Actinides.
- Reactions: Show similar reactivity within their respective series. Actinides are predominantly radioactive and are important in nuclear applications.
Applications and Importance of Group Understanding
Understanding the periodic table groups is essential in various fields:
- Chemistry: Predicting reaction outcomes, designing new materials, and understanding chemical bonding mechanisms.
- Materials Science: Developing new materials with specific properties, like conductors, semiconductors, and superconductors.
- Medicine: Understanding the roles of essential elements in biological processes and developing new drugs and treatments.
- Environmental Science: Analyzing environmental impacts of elements and pollutants.
- Industrial Processes: Optimizing industrial processes by using elements with desired properties.
Conclusion: Groups – The Key to Understanding the Periodic Table
The columns of the periodic table, known as groups or families, are a crucial aspect of understanding the chemical behavior of elements. The similar valence electron configurations within each group lead to predictable trends in properties and reactivities. This knowledge is vital for advancements in various fields, underscoring the importance of understanding the organization and properties of the periodic table's groups. The detailed exploration of each group highlights the remarkable diversity and interconnectedness of elements, forming the foundation of our understanding of the material world. The periodic table, with its groups and periods, continues to be an invaluable tool in scientific discovery and technological innovation.
Latest Posts
Latest Posts
-
Log X 3 Log X 1
Mar 15, 2025
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
-
Is Salt An Element Compound Or Mixture
Mar 15, 2025
-
How Many Millimeters Are In One Meter
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Columns On The Periodic Table Are Known As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
