How Many Atoms Are In Na
listenit
Mar 24, 2025 · 5 min read
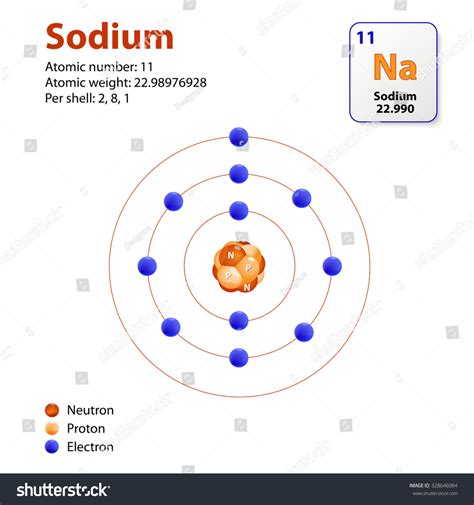
Table of Contents
How Many Atoms Are in a Grain of Salt (NaCl)? A Deep Dive into Avogadro's Number and Atomic Calculations
The seemingly simple question, "How many atoms are in a grain of salt?" opens a fascinating window into the world of chemistry, particularly the concepts of Avogadro's number, molar mass, and atomic structure. While we can't count atoms individually, we can use these fundamental principles to make remarkably accurate estimations. This article will guide you through the process, exploring the underlying concepts and providing a detailed calculation.
Understanding the Building Blocks: Atoms and Molecules
Before diving into the calculation, let's establish a foundational understanding of the key players: atoms and molecules.
-
Atoms: Atoms are the fundamental building blocks of matter. Each element (like sodium, Na, or chlorine, Cl) is composed of a unique type of atom. Atoms themselves are composed of protons, neutrons, and electrons. The number of protons defines the element.
-
Molecules: Molecules are formed when two or more atoms bond together. Table salt, chemically known as sodium chloride (NaCl), is an example of a molecule composed of one sodium atom and one chlorine atom.
The Key to the Calculation: Avogadro's Number
Avogadro's number (approximately 6.022 x 10²³) is a fundamental constant in chemistry. It represents the number of atoms or molecules in one mole of a substance. A mole is a unit of measurement used to quantify the amount of a substance. Think of it as a convenient way to deal with incredibly large numbers of atoms or molecules.
Determining the Molar Mass of NaCl
To calculate the number of atoms in a grain of salt, we first need to determine the molar mass of NaCl. This is the mass of one mole of NaCl in grams.
We find the molar mass by adding the atomic masses of sodium (Na) and chlorine (Cl). The atomic mass of an element is found on the periodic table and represents the average mass of an atom of that element, taking into account the different isotopes.
- Atomic mass of Na: Approximately 22.99 g/mol
- Atomic mass of Cl: Approximately 35.45 g/mol
Therefore, the molar mass of NaCl is:
22.99 g/mol + 35.45 g/mol = 58.44 g/mol
Estimating the Mass of a Grain of Salt
Now, we need to estimate the mass of a single grain of salt. This is where things get a little less precise, as the size of a "grain" can vary considerably. For our calculation, let's assume an average grain of salt has a mass of approximately 0.00005 grams (5 x 10⁻⁵ g). This is a reasonable estimate, but the actual mass could vary depending on the type of salt and its grain size.
Putting it All Together: The Calculation
We can now use the following steps to calculate the approximate number of atoms in our grain of salt:
-
Convert mass to moles: We'll use the molar mass of NaCl to convert the mass of our grain of salt into moles.
Moles of NaCl = (mass of grain of salt) / (molar mass of NaCl) Moles of NaCl = (5 x 10⁻⁵ g) / (58.44 g/mol) ≈ 8.55 x 10⁻⁷ mol
-
Calculate the number of NaCl molecules: We'll multiply the number of moles by Avogadro's number to find the number of NaCl molecules.
Number of NaCl molecules = (Moles of NaCl) x (Avogadro's Number) Number of NaCl molecules ≈ (8.55 x 10⁻⁷ mol) x (6.022 x 10²³ molecules/mol) ≈ 5.15 x 10¹⁷ molecules
-
Calculate the total number of atoms: Since each NaCl molecule contains two atoms (one Na and one Cl), we multiply the number of molecules by two to get the total number of atoms.
Total number of atoms ≈ (5.15 x 10¹⁷ molecules) x (2 atoms/molecule) ≈ 1.03 x 10¹⁸ atoms
Therefore, our estimate suggests that there are approximately 1.03 x 10¹⁸ atoms in a grain of salt weighing 0.00005 grams. Keep in mind that this is an approximation, and the actual number could vary depending on the size of the salt grain.
Sources of Error and Refinements
The calculation above contains several assumptions and potential sources of error:
- Grain size variation: The mass of a single grain of salt can vary significantly.
- Purity of the salt: The calculation assumes pure NaCl. Impurities would affect the molar mass and the final atom count.
- Avogadro's number approximation: Avogadro's number is an approximation itself.
To improve accuracy, one could:
- Weigh a larger sample: Weighing a larger sample of salt, say 1 gram, and dividing the result by the number of grains in that sample would yield a more precise average grain mass.
- Use a more precise balance: Using a high-precision analytical balance would improve the accuracy of the grain mass measurement.
- Employ advanced analytical techniques: Techniques such as X-ray diffraction or mass spectrometry could be used to determine the exact composition and therefore the precise molar mass of the salt sample.
Beyond a Grain of Salt: The Implications of Avogadro's Number
The ability to calculate the number of atoms in a macroscopic sample using Avogadro's number highlights the immense scale of the atomic world. This principle is crucial in various fields:
- Stoichiometry: Calculating reactant and product amounts in chemical reactions.
- Material science: Understanding the properties of materials based on their atomic structure and composition.
- Pharmacology: Determining drug dosages and effectiveness.
- Environmental science: Analyzing pollutant concentrations and their impact.
Conclusion
Calculating the number of atoms in a grain of salt is a seemingly simple problem that reveals the profound power of Avogadro's number and the fundamental concepts of chemistry. While the precise number will vary based on several factors, the calculation demonstrates the vast quantity of atoms present even in seemingly tiny amounts of matter. This understanding is fundamental to comprehending the world at a microscopic level and has far-reaching implications across various scientific disciplines. Understanding this process allows for a deeper appreciation of the scale of the universe at the atomic level and the importance of precise measurement in scientific investigation.
Latest Posts
Latest Posts
-
What Is 40 Percent Of 28
Mar 28, 2025
-
Do Nonmetals Gain Or Lose Electrons
Mar 28, 2025
-
Five Signs Of A Chemical Reaction
Mar 28, 2025
-
Square Root Of 192 Simplified Radical Form
Mar 28, 2025
-
12x14 Is How Many Square Feet
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
