How Many Atoms Are In H2so4
listenit
Mar 27, 2025 · 4 min read
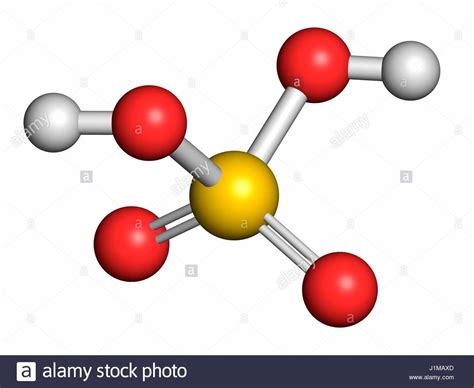
Table of Contents
How Many Atoms Are in H₂SO₄? A Deep Dive into Molecular Composition
Determining the number of atoms in a molecule like sulfuric acid (H₂SO₄) is fundamental to understanding chemistry. This seemingly simple question opens the door to exploring concepts like molecular formulas, molar mass, Avogadro's number, and the vast scale of the atomic world. Let's delve into the details and explore this topic comprehensively.
Understanding the Chemical Formula: H₂SO₄
The chemical formula H₂SO₄ tells us the type and number of atoms present in one molecule of sulfuric acid. Let's break it down:
- H: Represents a hydrogen atom. The subscript '2' indicates there are two hydrogen atoms in each sulfuric acid molecule.
- S: Represents a sulfur atom. There is one sulfur atom per molecule.
- O: Represents an oxygen atom. The subscript '4' signifies that there are four oxygen atoms in each molecule.
Calculating the Total Number of Atoms
Based on the chemical formula, we can easily calculate the total number of atoms in one molecule of H₂SO₄:
2 (hydrogen atoms) + 1 (sulfur atom) + 4 (oxygen atoms) = 7 atoms
Therefore, there are a total of seven atoms in a single molecule of sulfuric acid.
Expanding the Scale: Moles and Avogadro's Number
While understanding the number of atoms in a single molecule is crucial, we often work with macroscopic amounts of substances containing countless molecules. This is where the concept of moles and Avogadro's number comes into play.
A mole is a unit of measurement in chemistry representing a specific number of particles, whether atoms, molecules, ions, or other entities. This number is known as Avogadro's number, approximately 6.022 x 10²³. One mole of any substance contains Avogadro's number of particles.
So, one mole of H₂SO₄ contains 6.022 x 10²³ molecules. Since each molecule has 7 atoms, one mole of H₂SO₄ contains:
(6.022 x 10²³ molecules/mol) * (7 atoms/molecule) = 4.215 x 10²⁴ atoms
The Significance of Molar Mass
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's crucial for various chemical calculations, including determining the number of atoms in a given mass of a substance.
To calculate the molar mass of H₂SO₄, we need the atomic masses of its constituent elements:
- Hydrogen (H): approximately 1.008 g/mol
- Sulfur (S): approximately 32.06 g/mol
- Oxygen (O): approximately 16.00 g/mol
Therefore, the molar mass of H₂SO₄ is:
(2 * 1.008 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) = 98.08 g/mol
This means that 98.08 grams of H₂SO₄ contains 6.022 x 10²³ molecules, or 4.215 x 10²⁴ atoms.
Applications and Relevance
Understanding the number of atoms in H₂SO₄ and applying concepts like molar mass and Avogadro's number is essential in numerous applications, including:
- Stoichiometry: Calculating the amounts of reactants and products in chemical reactions. Knowing the number of atoms involved allows precise predictions of reaction outcomes.
- Titrations: Determining the concentration of solutions using volumetric analysis. The number of moles (and therefore atoms) in a given volume is fundamental to titration calculations.
- Industrial Processes: Controlling chemical reactions in industrial settings requires precise knowledge of the quantities of reactants, directly linked to the number of atoms involved.
- Environmental Science: Monitoring pollutant levels often involves analyzing the amounts of specific substances, requiring calculations based on the number of atoms or molecules present.
- Material Science: Designing new materials with specific properties often relies on understanding the arrangement and interactions of atoms at the molecular level.
Beyond the Basics: Isotopes and Atomic Mass
The calculations above utilize the average atomic masses of the elements. However, elements exist as isotopes, atoms of the same element with different numbers of neutrons. This means the actual number of atoms in a sample of H₂SO₄ can vary slightly depending on the isotopic composition. While this variation is generally small for most calculations, it's important to acknowledge for highly precise measurements.
Practical Considerations and Error Analysis
In practical experiments, determining the exact number of atoms in a sample of H₂SO₄ is challenging. Measurement errors inherent in weighing, volumetric analysis, and other techniques will always introduce some uncertainty. Understanding the sources of error and quantifying their impact is vital for accurate scientific work.
Conclusion: From Molecules to Macroscale
The seemingly simple question of how many atoms are in H₂SO₄ opens the door to a rich understanding of chemical principles. From the molecular level to the macroscopic scale, the concepts of molecular formulas, Avogadro's number, molar mass, and isotopic variations all play a role. Mastering these fundamental concepts provides a solid foundation for more advanced studies in chemistry and related scientific fields. The ability to accurately calculate the number of atoms in a given amount of substance is crucial for numerous applications across diverse scientific and industrial disciplines. Understanding these concepts enables us to bridge the gap between the microscopic world of atoms and the tangible world of chemical reactions and processes.
Latest Posts
Latest Posts
-
44 Is 55 Of What Number
Mar 30, 2025
-
How Does Price Discrimination Benefit Producers And Consumers
Mar 30, 2025
-
Which Element Is The Most Metallic
Mar 30, 2025
-
Do Srtrong Bases Completely Dissociate In Water
Mar 30, 2025
-
1000 Milliliters Is How Many Liters
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
