How Many Atoms Are In Ca
listenit
Mar 20, 2025 · 5 min read
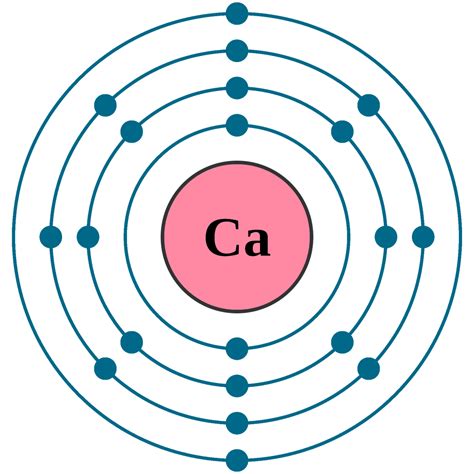
Table of Contents
How Many Atoms Are in a Ca (Calcium) Atom? Understanding Atomic Structure and Avogadro's Number
The question "How many atoms are in a Ca (Calcium) atom?" might seem deceptively simple. The answer, however, opens a door to a fascinating exploration of atomic structure, Avogadro's number, and the fundamental building blocks of matter. The seemingly straightforward query actually leads us to explore a more nuanced understanding of what constitutes a single atom versus a macroscopic quantity of calcium.
Understanding the Atom: A Single Unit of Calcium
At its most basic level, the answer is one. A single calcium atom contains only one calcium atom. This might seem trivial, but it's crucial to establish this foundational understanding before delving into larger quantities of calcium. Let's break down what characterizes this single calcium atom:
-
Protons: The calcium atom has 20 protons in its nucleus. Protons are positively charged particles that define the element. The number of protons is the atomic number, which is unique to each element. In this case, 20 protons identify the atom as calcium.
-
Neutrons: The number of neutrons can vary in calcium, leading to different isotopes. The most common isotope, Calcium-40, has 20 neutrons. Neutrons are neutral particles found in the nucleus along with protons.
-
Electrons: A neutral calcium atom also has 20 electrons. Electrons are negatively charged particles that orbit the nucleus in shells or energy levels. The arrangement of electrons determines the atom's chemical properties and reactivity.
Isotopes and Atomic Mass: Not All Calcium Atoms Are Created Equal
It's important to note that the term "calcium atom" encompasses different isotopes. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This means that while they all have 20 protons and are considered calcium, their mass differs slightly due to the varying number of neutrons. This variation in neutron count impacts the overall atomic mass of the atom.
While a single calcium atom contains only one calcium atom, understanding isotopes highlights that the mass of that single atom can vary slightly depending on its isotopic composition. The standard atomic mass of calcium listed on the periodic table (approximately 40.078 amu) is a weighted average of the masses of all naturally occurring calcium isotopes.
From Single Atom to Macroscopic Quantities: Avogadro's Number
The question becomes significantly more interesting when we shift our focus from a single calcium atom to a macroscopic sample of calcium, such as a piece of calcium metal. How many atoms are in, say, a gram of calcium? This is where Avogadro's number comes into play.
Avogadro's number (approximately 6.022 x 10<sup>23</sup>) is the number of atoms or molecules in one mole of a substance. A mole is a unit of measurement in chemistry that represents a specific amount of a substance based on its molar mass.
Calculating the Number of Atoms in a Given Mass of Calcium
To determine the number of calcium atoms in a given mass, we need to know the molar mass of calcium. The molar mass is approximately 40.078 grams per mole (g/mol). This means that one mole of calcium weighs approximately 40.078 grams.
Let's consider an example: How many atoms are in 10 grams of calcium?
-
Calculate the number of moles: Divide the mass of calcium (10 g) by the molar mass of calcium (40.078 g/mol):
10 g / 40.078 g/mol ≈ 0.249 moles
-
Calculate the number of atoms: Multiply the number of moles by Avogadro's number:
0.249 moles * 6.022 x 10<sup>23</sup> atoms/mol ≈ 1.50 x 10<sup>23</sup> atoms
Therefore, there are approximately 1.50 x 10<sup>23</sup> calcium atoms in 10 grams of calcium.
Practical Applications and Significance
Understanding the relationship between the number of atoms and the mass of a substance is crucial in various fields, including:
-
Chemistry: Stoichiometry, which involves calculating the amounts of reactants and products in chemical reactions, relies heavily on Avogadro's number and molar mass calculations.
-
Material Science: Determining the number of atoms in a material helps in understanding its properties and behavior. For instance, the number of atoms in a semiconductor affects its electrical conductivity.
-
Nuclear Physics: Understanding the number of atoms is critical in nuclear reactions and calculations involving radioactive decay.
-
Pharmacology and Medicine: Precise calculations involving Avogadro's number are vital in determining dosages of medications and understanding drug interactions at the molecular level.
Beyond Calcium: Generalizing the Concept
The principles discussed here for calcium apply to any element. To determine the number of atoms in a given mass of any element, follow these steps:
- Identify the element and find its molar mass from the periodic table.
- Determine the number of moles by dividing the given mass by the molar mass.
- Multiply the number of moles by Avogadro's number to calculate the number of atoms.
Conclusion: From One Atom to a Universe of Atoms
While a single calcium atom contains just one calcium atom, the vastness of Avogadro's number demonstrates the incredible scale at which atoms exist in macroscopic quantities of matter. Understanding this relationship between atomic structure, molar mass, and Avogadro's number is essential for comprehending the fundamental nature of matter and its behavior in diverse scientific and technological applications. The seemingly simple question of "How many atoms are in a Ca atom?" opens a doorway to a profound understanding of the world around us, built atom by atom. This knowledge extends far beyond simple calculations and into a deeper appreciation of the complexity and beauty of chemistry and the natural world. From a single atom to the vastness of the universe, the principles discussed here remain constant and fundamental to our understanding of the physical world.
Latest Posts
Latest Posts
-
How Many Resonance Structures Can Be Drawn For Ozone O3
Mar 21, 2025
-
What Percentage Is 5 Out Of 25
Mar 21, 2025
-
Number Of Valence Electrons In Aluminum
Mar 21, 2025
-
44 Is What Percent Of 50
Mar 21, 2025
-
What Percent Of 8 Is 20
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Ca . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
