How Many Resonance Structures Can Be Drawn For Ozone O3
listenit
Mar 21, 2025 · 5 min read
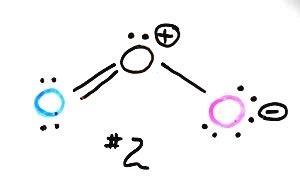
Table of Contents
How Many Resonance Structures Can Be Drawn for Ozone (O3)?
Ozone (O3), a crucial component of the Earth's stratosphere and a potent air pollutant at ground level, presents a fascinating case study in chemical bonding. Its structure isn't simply a linear arrangement of three oxygen atoms; instead, it exhibits resonance, a phenomenon where a single Lewis structure fails to fully represent the molecule's actual electronic distribution. Understanding the number of resonance structures for ozone and their significance is key to grasping its unique properties. This article delves into the intricacies of ozone's resonance, exploring the various ways to depict its bonding and explaining the implications of this resonance on its reactivity and overall characteristics.
Understanding Resonance Structures
Before diving into the specific case of ozone, let's establish a foundational understanding of resonance. Resonance is a concept in chemistry used to describe molecules where a single Lewis structure is insufficient to accurately represent the molecule's true structure. Instead, we use multiple Lewis structures, called resonance structures or canonical forms, to depict the delocalized electrons. These resonance structures are not different molecules; they represent different ways of depicting the same molecule, with the actual molecule being a hybrid or average of these contributing structures.
Key characteristics of resonance structures:
- They differ only in the placement of electrons (not atoms).
- They are not in equilibrium; the molecule does not switch between them.
- The true structure is a resonance hybrid, a weighted average of the contributing resonance structures.
- Resonance structures are connected by a double-headed arrow (↔).
Drawing Resonance Structures for Ozone (O3)
Ozone has a bent molecular geometry with a bond angle of approximately 117 degrees. This is significantly less than the 180 degrees expected for a linear molecule. To understand this, we need to consider its resonance structures. Let's explore the process of drawing these structures:
Step 1: Determine the total number of valence electrons.
Oxygen has 6 valence electrons, and with three oxygen atoms, the total number of valence electrons is 3 x 6 = 18.
Step 2: Arrange the atoms and connect them with single bonds.
We place the central oxygen atom and connect the other two oxygen atoms to it with single bonds. This uses 4 electrons (2 bonds x 2 electrons/bond).
Step 3: Distribute the remaining electrons as lone pairs.
We have 14 electrons remaining (18 - 4 = 14). We distribute these electrons as lone pairs around the terminal oxygen atoms to satisfy the octet rule (8 electrons around each atom). Each terminal oxygen will have 3 lone pairs (6 electrons).
Step 4: Check for octet rule satisfaction.
The central oxygen atom only has 6 electrons. To satisfy the octet rule, we need to create a double bond.
Step 5: Draw the resonance structures.
We can draw two equivalent resonance structures for ozone. In one structure, the double bond is between the central oxygen and one terminal oxygen. In the other, the double bond is between the central oxygen and the other terminal oxygen. These are shown below:
O=O-O ↔ O-O=O
Structure 1 Structure 2
These two structures are equivalent, meaning they contribute equally to the overall resonance hybrid. Therefore, there are two major resonance structures for ozone.
The Resonance Hybrid
The actual ozone molecule is not represented by either of the two individual resonance structures. It's best described as a resonance hybrid, a weighted average of the two contributing structures. In this hybrid, the electrons are delocalized across the three oxygen atoms. This means the electron density is spread out, rather than concentrated in a single bond or lone pair. This delocalization is crucial in explaining ozone's properties.
Bond order:
The concept of bond order helps understand the nature of the bonding in the ozone resonance hybrid. Bond order is the number of chemical bonds between a pair of atoms. In ozone, the bond order is calculated as the total number of bonds divided by the number of bond locations. In our case, we have a total of 4 bonds (2 double bonds and 2 single bonds) divided by 2 bonds between O-O atoms. The bond order is 1.5. This indicates that each O-O bond is intermediate between a single and a double bond, which explains the shorter bond length than a typical O-O single bond.
Implications of Resonance on Ozone's Properties
The resonance in ozone leads to several of its important properties:
- Bond Length: The O-O bond length in ozone is intermediate between a single and a double bond. This is because the electron density is delocalized across both bonds.
- Reactivity: The delocalized electrons make ozone a powerful oxidizing agent. The molecule is more reactive than one might expect based on simple Lewis structures.
- Polarity: While the two resonance structures appear symmetrical, the actual molecule exhibits a small dipole moment due to the asymmetrical distribution of electrons in the resonance hybrid.
- Stability: Resonance stabilization enhances the overall stability of the ozone molecule. This stability, however, is relative; ozone is still a reactive molecule compared to, for instance, oxygen (O2).
Beyond the Two Major Resonance Structures: Minor Contributing Structures
While the two major resonance structures are sufficient to represent ozone’s bonding behavior accurately for most purposes, it is theoretically possible to draw additional, less significant resonance structures. These structures would involve charge separation or violations of the octet rule. They contribute minimally to the overall resonance hybrid and are generally less stable than the two primary structures.
For example, one could draw structures with more than one double bond or with significant charge separation. However, these structures are less likely to occur because they involve a higher energy state and would contribute very little to the resonance hybrid’s overall character.
Conclusion
In summary, while several resonance structures can be conceptually drawn for ozone, two major equivalent resonance structures best represent the molecule's true bonding. These structures highlight the delocalization of electrons and the intermediate bond order between the oxygen atoms. This delocalization significantly affects ozone's reactivity, bond length, polarity, and overall stability, impacting its role in atmospheric chemistry and its industrial applications. Understanding ozone's resonance is crucial for appreciating its multifaceted chemical behavior and its diverse impact on the environment and human activities. The application of resonance theory allows us to move beyond simplistic Lewis structures and gain a more accurate and complete understanding of the intricacies of molecular bonding in a remarkably important molecule.
Latest Posts
Latest Posts
-
1000 Ml Equals How Many Liters
Mar 28, 2025
-
Inverse Of X 2 X 1
Mar 28, 2025
-
What Is The Mass Number Of Magnesium
Mar 28, 2025
-
How Many Symmetrical Lines Does A Rectangle Have
Mar 28, 2025
-
What Is The Percent Of 0 125
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Resonance Structures Can Be Drawn For Ozone O3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
