How Many Atoms Are In A Single Molecule Of Water
listenit
Mar 17, 2025 · 5 min read
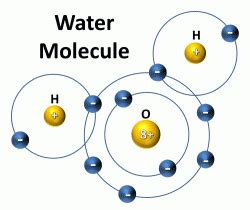
Table of Contents
How Many Atoms Are in a Single Molecule of Water? A Deep Dive into Atomic Structure
The seemingly simple question, "How many atoms are in a single molecule of water?" opens a door to a fascinating exploration of chemistry, atomic structure, and the very building blocks of our world. While the answer itself is straightforward, understanding why that answer is correct requires delving into the fundamental concepts of molecules and atoms. This article will not only answer this question but also explore related concepts to provide a comprehensive understanding of the topic.
Understanding Atoms: The Fundamental Building Blocks
Before we can count atoms in a water molecule, we need to grasp the concept of an atom itself. Atoms are the smallest units of matter that retain the chemical properties of an element. Each atom is composed of a nucleus containing protons (positively charged) and neutrons (neutral charge), surrounded by a cloud of electrons (negatively charged). The number of protons in an atom's nucleus defines its atomic number and determines what element it is. For example, all atoms with one proton are hydrogen, while those with eight protons are oxygen.
Isotopes and Atomic Mass
While the number of protons determines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. For example, hydrogen has three isotopes: protium (one proton, zero neutrons), deuterium (one proton, one neutron), and tritium (one proton, two neutrons). The atomic mass of an element is the average mass of its isotopes, taking into account their relative abundance. This is why atomic masses on the periodic table are not whole numbers.
Molecules: Atoms Bonding Together
Atoms rarely exist in isolation. They tend to interact and bond with each other to form molecules. A molecule is a group of two or more atoms held together by chemical bonds. These bonds are formed through the interaction of electrons in the outermost shells of the atoms, seeking stability by achieving a full electron shell. There are several types of chemical bonds, but the most common are covalent bonds, where atoms share electrons.
Covalent Bonds in Water Molecules
Water (H₂O) is a classic example of a molecule formed by covalent bonds. Each hydrogen atom shares one electron with the oxygen atom, forming a stable molecule. This sharing creates a strong bond that holds the atoms together. The oxygen atom, being more electronegative than hydrogen, attracts the shared electrons more strongly, leading to a polar molecule with a slightly negative charge near the oxygen and slightly positive charges near the hydrogens. This polarity is crucial for many of water's unique properties.
Answering the Question: Atoms in a Water Molecule
Now, armed with a basic understanding of atoms and molecules, we can finally answer the central question: A single molecule of water (H₂O) contains three atoms. Two of these atoms are hydrogen (H), and one is oxygen (O). The chemical formula H₂O clearly indicates this: the subscript "2" next to the H signifies two hydrogen atoms.
Exploring the Significance of Water's Atomic Composition
The simple composition of water – two hydrogen atoms and one oxygen atom – belies its immense importance. This seemingly simple molecule is essential for life as we know it. Its unique properties, largely stemming from its polar nature and hydrogen bonding, make it crucial for numerous biological processes:
-
Solvent: Water's polarity allows it to dissolve many substances, making it an excellent solvent for biological reactions. It acts as a medium for transporting nutrients and removing waste products in living organisms.
-
Temperature Regulation: Water's high specific heat capacity helps regulate temperature fluctuations, preventing drastic changes that could harm living organisms. This is vital for maintaining stable internal temperatures in organisms.
-
Cohesion and Adhesion: Water molecules stick together (cohesion) due to hydrogen bonding, and they also stick to other surfaces (adhesion). This allows water to be transported against gravity in plants and contributes to surface tension, which is vital for many aquatic organisms.
-
Density Anomaly: Water's unusual density anomaly (ice is less dense than liquid water) is essential for aquatic life. This allows ice to float, insulating the water below and preventing it from freezing solid, ensuring the survival of aquatic organisms during winter.
Beyond the Basics: Isotopes and Variations in Water
While the standard water molecule is H₂O, variations exist due to the presence of hydrogen isotopes. These variations, although rare, have important implications in various scientific fields:
-
Heavy Water (D₂O): This is water where both hydrogen atoms are deuterium (²H or D). It's denser than regular water and is used in nuclear reactors as a neutron moderator.
-
Tritiated Water (T₂O): This is water where both hydrogen atoms are tritium (³H or T). It's radioactive and is used as a tracer in various scientific experiments.
-
Semi-heavy water (HDO): This contains one atom of deuterium and one atom of protium. It's naturally present in small amounts and serves as a key component in understanding isotopic variations in water cycles.
These isotopic variations in water highlight the complexities that arise even with the seemingly simple molecule H₂O. The presence and distribution of these isotopes provide valuable insights into various hydrological and environmental processes.
Conclusion: The Importance of Understanding Atomic Composition
The question of how many atoms are in a water molecule, while seemingly straightforward, serves as a gateway to understanding fundamental concepts in chemistry and the importance of atomic structure. The simple answer – three atoms – unveils a world of complexity. The properties of water, crucial for life itself, arise directly from its molecular structure and the arrangement of its atoms. Understanding this simple molecule allows us to appreciate the intricate interplay between atoms, molecules, and the larger world around us. Further exploring the isotopic variations and the impact on various scientific fields further reinforces the importance of comprehending the basic building blocks of matter and their interactions. The journey from a simple question to a deeper understanding of the natural world is a testament to the power of scientific inquiry and the beauty of the universe's fundamental building blocks.
Latest Posts
Latest Posts
-
What Is The Square Root Of 145
Mar 17, 2025
-
Is 2 3 Same As 3 4
Mar 17, 2025
-
What Is 3 And 1 8 As A Decimal
Mar 17, 2025
-
Lowest Common Multiple Of 20 And 30
Mar 17, 2025
-
What Is Gcf Of 36 And 54
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Single Molecule Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
