How Is Density Affected By Temperature
listenit
Mar 23, 2025 · 5 min read
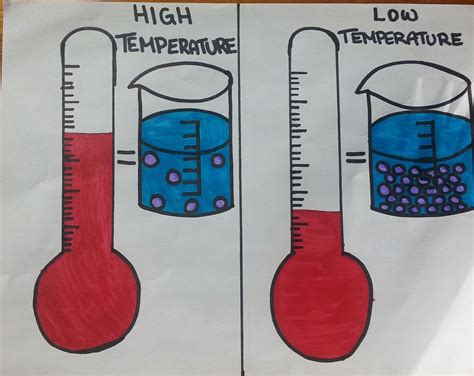
Table of Contents
How is Density Affected by Temperature? A Comprehensive Guide
Density, a fundamental physical property, describes how much mass is packed into a given volume. It's calculated simply as mass divided by volume (ρ = m/V). While seemingly straightforward, density's relationship with temperature is surprisingly complex and varies significantly depending on the material's state (solid, liquid, or gas). Understanding this relationship is crucial in numerous fields, from engineering and materials science to meteorology and oceanography. This comprehensive guide delves deep into the intricate dance between temperature and density.
The General Trend: Temperature's Impact on Density
Generally, an increase in temperature leads to a decrease in density, and vice-versa. This holds true for most substances, but there are notable exceptions. Let's explore why this is the case:
Thermal Expansion: The Driving Force
The primary reason behind the inverse relationship between temperature and density is thermal expansion. As temperature rises, the particles within a substance gain kinetic energy. This increased energy causes them to move more vigorously, leading to an increase in the average distance between particles. Consequently, the substance expands, occupying a larger volume. Since the mass remains constant, the density (mass/volume) decreases.
This effect is most pronounced in gases. Gases are highly compressible, and their particles are relatively far apart. A small temperature increase significantly expands the volume, resulting in a substantial decrease in density. Liquids exhibit thermal expansion as well, but the effect is less dramatic due to the closer proximity of their particles. Solids typically experience the least amount of thermal expansion, with their density changes being the smallest in response to temperature fluctuations.
Exceptions to the Rule: Water's Anomalous Behavior
While the general trend is a decrease in density with increasing temperature, water stands out as a significant exception. Water exhibits an anomalous behavior in its density-temperature relationship.
-
Between 0°C and 4°C: As water cools from 4°C to 0°C, it expands instead of contracting, resulting in a decrease in density. This is due to the unique hydrogen bonding structure of water molecules. At temperatures below 4°C, the hydrogen bonds rearrange into a less dense, ice-like structure. This is why ice floats on water; its density is lower than liquid water at 0°C.
-
Above 4°C: Water follows the general trend, with its density decreasing as the temperature increases.
This unusual behavior of water is crucial for aquatic life. The lower density of ice prevents bodies of water from freezing solid, allowing aquatic organisms to survive during winter.
Density Changes in Different States of Matter
The impact of temperature on density is profoundly different for solids, liquids, and gases. Let's examine each state individually:
Solids: Minimal Density Changes
Solids have a relatively fixed structure with particles closely packed together. Therefore, thermal expansion in solids is usually quite small. The change in density with temperature in solids is generally linear over a moderate temperature range. However, significant temperature changes can lead to phase transitions (e.g., melting), drastically altering the density.
Liquids: Moderate Density Changes
Liquids are more compressible than solids, leading to more noticeable density changes with temperature. The relationship between density and temperature in liquids is often more complex than in solids, sometimes deviating from linearity. The strength of intermolecular forces plays a crucial role in determining the extent of density change.
Gases: Significant Density Changes
Gases exhibit the most significant density changes in response to temperature variations. This is because gas particles are widely spaced and their interactions are minimal. The ideal gas law (PV = nRT) describes the relationship between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). From this, we can derive the relationship between density and temperature for an ideal gas: density is inversely proportional to temperature at constant pressure.
Factors Influencing Density-Temperature Relationship
Beyond the state of matter, several other factors can influence how temperature affects density:
-
Pressure: Pressure significantly impacts the density of gases and, to a lesser extent, liquids and solids. Increased pressure forces particles closer together, leading to a higher density regardless of temperature.
-
Composition: The chemical composition of a substance greatly influences its thermal expansion and, consequently, its density-temperature relationship. Different substances have varying intermolecular forces and atomic structures, resulting in diverse responses to temperature changes.
-
Impurities: The presence of impurities in a substance can alter its density-temperature relationship. Impurities can affect the intermolecular interactions and the overall structure, leading to deviations from the expected behavior.
-
Phase Transitions: Phase transitions (melting, boiling, sublimation) involve dramatic changes in density. These transitions are accompanied by significant structural rearrangements, resulting in abrupt density changes at specific temperatures.
Applications and Importance
Understanding the density-temperature relationship is crucial in many applications:
-
Meteorology: Density differences in air masses due to temperature variations drive weather patterns. Warm, less dense air rises, while cool, denser air sinks, creating convection currents that influence wind and precipitation.
-
Oceanography: Temperature variations in the ocean create density gradients that drive ocean currents. These currents play a critical role in global heat distribution and marine ecosystems.
-
Materials Science: The density-temperature relationship is crucial in the design and selection of materials for various applications. The thermal expansion properties of materials must be considered to ensure structural integrity and functionality under varying temperature conditions.
-
Engineering: Understanding thermal expansion is essential in engineering design to account for dimensional changes in structures and components due to temperature fluctuations.
-
Food Science: Density changes during food processing and storage are important factors to consider for quality control and shelf-life.
Conclusion: A Complex Yet Crucial Relationship
The relationship between density and temperature is a complex one, influenced by multiple factors. While the general trend is an inverse relationship, exceptions exist, most notably in the case of water. A thorough understanding of this relationship is vital across numerous scientific and engineering disciplines. From predicting weather patterns to designing robust structures, appreciating the subtle nuances of how temperature impacts density is paramount for success in many fields. Further research continues to refine our understanding of this fundamental property and its implications in various contexts.
Latest Posts
Latest Posts
-
What Does The Atomic Number Tell Us About An Atom
Mar 25, 2025
-
What Element Is Always Present In An Organic Compound
Mar 25, 2025
-
What Is 25 As A Decimal
Mar 25, 2025
-
What Is The Decimal Of 4 6
Mar 25, 2025
-
How To Write Electron Configuration For Ions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Is Density Affected By Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
