What Does The Atomic Number Tell Us About An Atom
listenit
Mar 25, 2025 · 6 min read
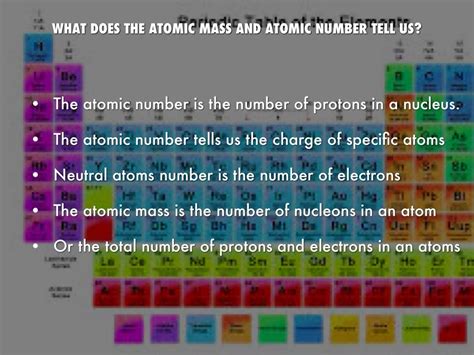
Table of Contents
What Does the Atomic Number Tell Us About an Atom?
The atomic number, a seemingly simple numerical value, holds the key to understanding the fundamental nature of an atom. It's more than just a label; it dictates the atom's identity, its chemical properties, and its behavior within the wider world of chemistry and physics. This article delves deep into the significance of the atomic number, exploring its implications for atomic structure, chemical reactivity, and isotopic variations.
The Atomic Number: A Defining Characteristic
The atomic number, represented by the symbol Z, is the number of protons found in the nucleus of an atom. This seemingly simple definition has profound consequences. It's the single most crucial piece of information that defines an element. All atoms of a particular element possess the same atomic number. For instance, all hydrogen atoms have an atomic number of 1, meaning each possesses one proton in its nucleus. Similarly, all oxygen atoms have an atomic number of 8, indicating eight protons. This unwavering consistency makes the atomic number a cornerstone of the periodic table, which organizes elements based on their increasing atomic numbers.
The Connection Between Atomic Number and Identity
The atomic number uniquely identifies an element. No two elements share the same atomic number. This is because the number of protons dictates the fundamental characteristics of the element, including its electron configuration, which in turn determines its chemical behavior. Elements with differing atomic numbers exhibit distinct physical and chemical properties. For example, hydrogen, with Z = 1, is a highly reactive gas, while oxygen, with Z = 8, is a reactive gas essential for respiration. This stark contrast highlights how the atomic number fundamentally shapes the nature of an element.
Atomic Structure and the Atomic Number
The atomic number is intrinsically linked to the atom's structure. Since an atom is electrically neutral, the number of protons (Z) must equal the number of electrons. This balance of positive and negative charges maintains the atom's overall neutrality. Therefore, the atomic number indirectly dictates the number of electrons orbiting the nucleus. These electrons are arranged in specific energy levels or shells, and their configuration determines the atom's chemical reactivity.
Electron Configuration and Chemical Properties
The arrangement of electrons in different energy levels, known as the electron configuration, is directly influenced by the atomic number. Electrons occupy these levels according to specific rules, filling lower energy levels before moving to higher ones. The outermost electrons, called valence electrons, are primarily responsible for the atom's chemical behavior. Atoms tend to react with other atoms to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons. This drive for stability dictates how atoms bond with each other, forming molecules and compounds. The atomic number, by determining the number of electrons and, consequently, the electron configuration, directly dictates this chemical reactivity.
Isotopes and the Atomic Number: A Closer Look
While the atomic number defines the element, the number of neutrons in the nucleus can vary, leading to the existence of isotopes. Isotopes are atoms of the same element (same atomic number) that have different numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties. For instance, carbon-12 and carbon-14 are both isotopes of carbon (Z = 6), differing only in their neutron count (6 and 8, respectively). The atomic number remains constant; it's the neutron count that distinguishes them.
Understanding isotopes is crucial in various fields, including medicine (radioactive isotopes in medical imaging), geology (radioactive dating), and environmental science (tracing pollutants). The atomic number provides the essential framework for recognizing and understanding these isotopic variations.
The Periodic Table: A Testament to Atomic Number
The periodic table, a cornerstone of chemistry, is arranged according to increasing atomic number. This arrangement reveals periodic trends in physical and chemical properties. Elements with similar electron configurations and, thus, similar chemical behaviors, are grouped together in columns (groups or families). Rows (periods) reflect the filling of successive electron shells. The table's structure is a direct consequence of the unique identity conferred by the atomic number.
Predicting Properties Based on Atomic Number
The atomic number allows us to predict certain properties of an element. For example, elements in the same group tend to exhibit similar reactivity due to the similarity in their valence electron configurations. By knowing the atomic number, we can infer its position on the periodic table and, consequently, predict its general properties. This predictive power of the atomic number simplifies the understanding of the vast array of elements and their interactions.
Beyond the Basics: Advanced Implications
The atomic number’s importance extends beyond basic atomic structure and chemical reactivity. It plays a significant role in various advanced concepts:
Nuclear Chemistry and Nuclear Reactions
Nuclear reactions involve changes in the atom's nucleus, including changes in the number of protons and neutrons. The atomic number is crucial in identifying the elements involved in these reactions and in predicting the products. Nuclear fission and fusion, processes vital in energy production and nuclear weapons, are governed by changes in atomic numbers and the associated release of energy.
Spectroscopy and Atomic Fingerprints
Each element has a unique atomic spectrum, a pattern of light emitted or absorbed when electrons transition between energy levels. This spectrum is directly related to the atom's electron configuration, which is determined by the atomic number. Spectroscopy, the study of atomic spectra, allows us to identify elements based on their unique "fingerprint" of emitted or absorbed light. This technique is crucial in astronomy, analyzing the composition of stars and other celestial bodies.
Materials Science and Engineering
The atomic number plays a critical role in materials science and engineering, influencing the properties of materials. The arrangement and interactions of atoms, governed by their atomic numbers, determine the physical and mechanical properties of materials, from their strength and conductivity to their malleability and ductility. This knowledge is used to design and develop materials with specific properties for various applications.
Conclusion: The Universal Significance of the Atomic Number
The atomic number, while seemingly a simple number, is a fundamental characteristic of an atom. It acts as a unique identifier, dictating the atom’s identity, structure, and chemical behavior. From the periodic table's organization to predicting chemical reactivity and understanding nuclear reactions, the atomic number's implications are vast and profound. Its role in diverse fields, from chemistry and physics to medicine and materials science, emphasizes its universal significance in our understanding of matter and its properties. It is the cornerstone upon which our understanding of the chemical world is built. Its simplicity belies its immense power in explaining the complexity of the universe at its most fundamental level.
Latest Posts
Latest Posts
-
What Is 13 16 As A Decimal
Mar 26, 2025
-
How Many Ounces Are In 3 4
Mar 26, 2025
-
Weight Of One Cubic Metre Of Water
Mar 26, 2025
-
What Is The Measure Of Angle A
Mar 26, 2025
-
What Is 60 Percent Of 800
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Does The Atomic Number Tell Us About An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
