How Is A Modern Periodic Table Organized
listenit
Mar 29, 2025 · 6 min read
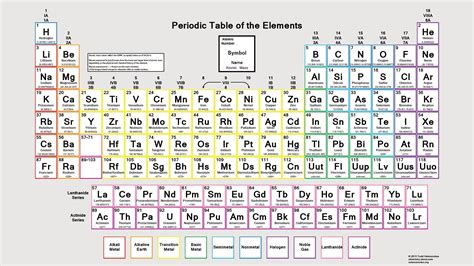
Table of Contents
How is a Modern Periodic Table Organized?
The modern periodic table, a cornerstone of chemistry, is a marvel of organization, neatly arranging the chemical elements in a way that reveals their properties and relationships. Understanding its organization is key to understanding chemistry itself. This comprehensive guide will delve into the intricacies of the modern periodic table, explaining its structure, the principles behind its arrangement, and the valuable information it provides.
The Fundamental Principle: Atomic Number
The periodic table's organization hinges on a single, crucial property: the atomic number. The atomic number represents the number of protons in an atom's nucleus. It is this number that uniquely identifies each element. Hydrogen (H), with one proton, has an atomic number of 1. Helium (He), with two protons, has an atomic number of 2, and so on. Crucially, the periodic table arranges elements in ascending order of their atomic numbers. This is the fundamental principle that governs the entire structure.
Periods: Horizontal Rows of Elements
The periodic table is arranged in horizontal rows called periods. Each period corresponds to a principal energy level (or electron shell) in an atom. The first period, containing only hydrogen and helium, represents the filling of the first energy level. The second period has eight elements, reflecting the filling of the second energy level. The number of elements in each period varies, increasing as we move down the table. This variation is due to the increasing complexity of electron shells and sub-shells.
Understanding Electron Shells and Subshells
Atoms have electrons organized into shells, with each shell capable of holding a specific maximum number of electrons. Furthermore, shells are subdivided into subshells (s, p, d, and f), each capable of holding a specific number of electrons. The filling of these subshells dictates the periodic table's structure, with different blocks of elements corresponding to the filling of specific subshells.
Groups: Vertical Columns of Elements
The vertical columns of the periodic table are called groups or families. Elements within the same group share similar chemical properties because they have the same number of valence electrons. Valence electrons are the electrons in the outermost energy level of an atom; these electrons are primarily involved in chemical bonding and reactions. For example, all elements in Group 1 (alkali metals) have one valence electron, and all elements in Group 18 (noble gases) have eight valence electrons (except helium, which has two).
Key Groups and Their Properties:
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron. They readily lose this electron to form +1 ions.
- Group 2 (Alkaline Earth Metals): Reactive metals with two valence electrons, forming +2 ions.
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions.
- Group 18 (Noble Gases): Inert gases with a full valence shell (except helium). They are extremely unreactive due to their stable electron configuration.
- Transition Metals (Groups 3-12): Metals with variable oxidation states, meaning they can lose different numbers of electrons to form ions with different charges. This contributes to their diverse chemical properties and the formation of numerous compounds.
- Inner Transition Metals (Lanthanides and Actinides): These elements are placed separately at the bottom of the table for convenience, but they belong to periods 6 and 7 respectively. They are characterized by the filling of the f-subshell.
Blocks: Regions Representing Subshell Filling
The periodic table can also be divided into blocks based on the type of subshell being filled. These blocks are named after the subshells:
- s-block: Contains Groups 1 and 2 (alkali and alkaline earth metals) and helium. The outermost s-subshell is being filled in these elements.
- p-block: Contains Groups 13-18. The outermost p-subshell is being filled in these elements. This block includes a wide variety of elements, including nonmetals, metalloids, and some metals.
- d-block: Contains the transition metals (Groups 3-12). The d-subshell is being filled in these elements.
- f-block: Contains the inner transition metals (lanthanides and actinides). The f-subshell is being filled in these elements.
Metalloids, Metals, and Nonmetals: A Tripartite Division
The periodic table also reveals a broader classification of elements into three main categories based on their physical and chemical properties:
- Metals: Located on the left side and center of the table, metals are generally good conductors of heat and electricity, malleable (can be hammered into sheets), ductile (can be drawn into wires), and have a lustrous appearance. They tend to lose electrons to form positive ions.
- Nonmetals: Located on the right side of the table, nonmetals are generally poor conductors of heat and electricity, brittle, and lack metallic luster. They tend to gain electrons to form negative ions.
- Metalloids (Semimetals): Located along a zigzag line separating metals and nonmetals, metalloids have intermediate properties. They exhibit some characteristics of both metals and nonmetals, and their conductivity can vary depending on conditions. This makes them crucial in semiconductor technology.
Trends in the Periodic Table: Periodicity
The arrangement of elements in the periodic table leads to predictable trends in their properties, a phenomenon known as periodicity. These trends are observed across periods and down groups. Some of the most significant periodic trends include:
- Atomic Radius: The size of an atom generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increased nuclear charge).
- Ionization Energy: The energy required to remove an electron from an atom generally decreases down a group (due to increased atomic radius and shielding) and increases across a period (due to increased nuclear charge).
- Electronegativity: The ability of an atom to attract electrons in a chemical bond generally decreases down a group (due to increased atomic radius) and increases across a period (due to increased nuclear charge).
- Electron Affinity: The energy change associated with gaining an electron generally decreases down a group and increases across a period, although there are some exceptions.
The Significance of the Modern Periodic Table
The modern periodic table is far more than just a list of elements; it's a powerful tool that allows chemists to:
- Predict the properties of elements: Knowing an element's position on the table allows chemists to predict its properties with reasonable accuracy.
- Understand chemical bonding and reactivity: The arrangement of electrons, revealed by the table, is crucial for understanding how elements bond and react with each other.
- Develop new materials and technologies: The table provides a framework for designing new materials with specific properties, driving innovation in various fields.
- Study nuclear chemistry: The table provides insights into the stability and radioactivity of elements, which are essential aspects of nuclear chemistry.
Conclusion: A Dynamic and Evolving Tool
The modern periodic table, while seemingly static, is a dynamic and constantly evolving tool. As our understanding of the atom and its properties deepens, our understanding of the periodic table also evolves. New elements are constantly being discovered and added, and our understanding of the relationships between elements continues to refine. However, the fundamental principles behind its organization—atomic number, electron configuration, and periodic trends—remain unshaken, making it an indispensable tool for anyone seeking to understand the building blocks of our universe. The ability to predict and understand the chemical behavior of elements is a direct consequence of this elegant organization, solidifying the periodic table’s place as a central pillar of modern chemistry and beyond.
Latest Posts
Latest Posts
-
What Is The Driving Force Behind Plate Movement
Mar 31, 2025
-
What Is 3 11 As A Decimal
Mar 31, 2025
-
What Is The Square Root Of 841
Mar 31, 2025
-
Chance Of Rolling Two Nat 20s
Mar 31, 2025
-
Does A Gas Have A Definite Shape And Volume
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Is A Modern Periodic Table Organized . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
