How Elements Are Arranged In The Modern Periodic Table
listenit
Apr 01, 2025 · 6 min read
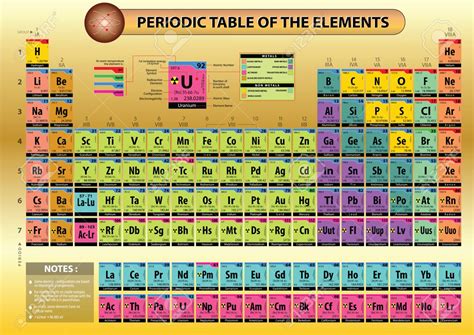
Table of Contents
How Elements Are Arranged in the Modern Periodic Table: A Deep Dive
The modern periodic table, a cornerstone of chemistry, isn't just a random arrangement of elements. It's a meticulously organized system reflecting the fundamental properties and behaviors of atoms. Understanding its structure unlocks a deeper appreciation of the intricate relationships between different elements and their chemical reactions. This article delves into the principles behind the arrangement, exploring the key factors that dictate an element's position and the valuable insights this organization provides.
The Genesis of the Periodic Table: Mendeleev's Vision
Before the modern table, several scientists attempted to categorize elements. However, Dmitri Mendeleev's 1869 periodic table stands out as a pivotal breakthrough. He arranged the known elements in order of increasing atomic weight, observing recurring patterns in their properties. This led to his revolutionary arrangement, where elements with similar properties were placed in the same vertical column, or group. Mendeleev's genius lay not only in arranging the known elements but also in predicting the existence and properties of undiscovered elements based on gaps in his table. These predictions were later confirmed, solidifying the table's validity.
Beyond Atomic Weight: The Modern Refinement
While Mendeleev's table was groundbreaking, it had limitations. The arrangement based solely on atomic weight occasionally caused inconsistencies. The discovery of isotopes – atoms of the same element with different numbers of neutrons – further complicated matters. The modern periodic table's organization rests on a more fundamental property: atomic number.
The atomic number represents the number of protons in an atom's nucleus. This is a unique identifier for each element, unlike atomic weight, which can vary slightly among isotopes. This fundamental shift transformed the periodic table into a more precise and accurate representation of elemental relationships.
The Structure: Periods and Groups
The modern periodic table is a grid consisting of:
-
Periods (Rows): Elements are arranged horizontally in periods. Each period corresponds to a principal energy level (or shell) in an atom. The first period has only two elements (hydrogen and helium), reflecting the filling of the lowest energy level. Subsequent periods contain more elements as higher energy levels accommodate more electrons.
-
Groups (Columns): Elements are arranged vertically in groups, also known as families. Elements within the same group share similar chemical properties because they have the same number of valence electrons. Valence electrons are the electrons in the outermost shell and are primarily responsible for an element's reactivity. For instance, elements in Group 1 (alkali metals) all have one valence electron, leading to their high reactivity.
The Significance of Valence Electrons
The similarity in chemical behavior within a group stems directly from the shared number of valence electrons. These electrons participate in chemical bonding, dictating how an element interacts with other elements. For example:
-
Group 18 (Noble Gases): These elements have a full outer electron shell (eight valence electrons, except for helium with two). This stable electron configuration makes them extremely unreactive and chemically inert.
-
Group 17 (Halogens): These elements have seven valence electrons, meaning they readily gain one electron to achieve a stable, noble gas configuration. This explains their high reactivity and tendency to form -1 ions.
-
Group 1 (Alkali Metals): With one valence electron, these metals easily lose that electron to form +1 ions, resulting in their high reactivity with water and other substances.
The Blocks: s, p, d, and f
The periodic table is further divided into blocks based on the subshells where the valence electrons reside:
-
s-block: This block includes Groups 1 and 2 (alkali and alkaline earth metals). These elements have their valence electrons in the s subshell.
-
p-block: This block spans Groups 13 to 18. The valence electrons reside in the p subshell. This block contains a diverse range of elements, including nonmetals, metalloids, and some metals.
-
d-block: This block encompasses Groups 3 to 12, often called the transition metals. Their valence electrons are in the d subshell, leading to variable oxidation states and complex ion formation. Many transition metals are known for their colorful compounds and catalytic properties.
-
f-block: These elements, located separately at the bottom of the table, are called the inner transition metals. They are divided into the lanthanides and actinides. Their valence electrons occupy the f subshell. Many actinides are radioactive.
Periodic Trends: Predicting Elemental Behavior
The periodic table's organization allows us to predict trends in various elemental properties. These trends arise from the systematic changes in atomic structure as we move across periods and down groups:
-
Atomic Radius: Generally, atomic radius increases down a group (due to the addition of electron shells) and decreases across a period (due to increasing nuclear charge).
-
Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally decreases down a group and increases across a period.
-
Electron Affinity: The energy change when an atom gains an electron. Electron affinity generally increases across a period and decreases down a group (with some exceptions).
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
Understanding these trends is crucial in predicting the reactivity and bonding behavior of elements. For example, the high electronegativity of halogens explains their tendency to attract electrons and form negative ions.
Beyond the Basics: Exceptions and Nuances
While the periodic table provides a powerful framework for understanding elemental properties, it's important to acknowledge exceptions and nuances. Some elements don't perfectly follow the predicted trends. This is often due to the complexities of electron-electron interactions and relativistic effects in heavier elements.
The Role of Electron Configuration
The electron configuration of an element—the arrangement of electrons in its energy levels and subshells—underlies its position and properties in the periodic table. While the general trends are easily observed, irregularities can occur due to the specific electron configurations. For instance, some transition metals exhibit unexpected oxidation states due to the involvement of d electrons in bonding.
Relativistic Effects
In heavier elements, relativistic effects – changes in electron behavior due to their high speeds—can significantly influence their properties. These effects can lead to deviations from expected trends, particularly in atomic radii and ionization energies of elements with high atomic numbers.
The Periodic Table: An Ongoing Story
The periodic table is not a static entity. Our understanding of its intricacies continues to evolve as new elements are synthesized and our knowledge of atomic structure deepens. The discovery of new elements, particularly superheavy elements, poses unique challenges and opportunities in refining our understanding of the periodic system.
Conclusion: The Power of Organization
The modern periodic table is a testament to the power of scientific inquiry and the beauty of underlying order in nature. Its organization, based on atomic number and electron configuration, provides a powerful framework for understanding the properties and behaviors of elements. From predicting chemical reactions to synthesizing new materials, the periodic table serves as an indispensable tool in chemistry and related fields. It is a dynamic and evolving system, continuously refined and enriched by ongoing research, reminding us that our understanding of the universe is constantly unfolding. The seemingly simple grid holds within it the key to unlocking the secrets of matter itself, a testament to the elegance and power of scientific discovery.
Latest Posts
Latest Posts
-
Density Of Water At 4 C
Apr 02, 2025
-
Select The Graphs That Have An Equation With A 0
Apr 02, 2025
-
Name The 2 Functional Groups In Amino Acids
Apr 02, 2025
-
A Reaction Has A Standard Free Energy Change Of
Apr 02, 2025
-
How To Find Amplitude Period And Phase Shift
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Elements Are Arranged In The Modern Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
