How Does An Ion Differ From An Atom
listenit
Apr 01, 2025 · 6 min read
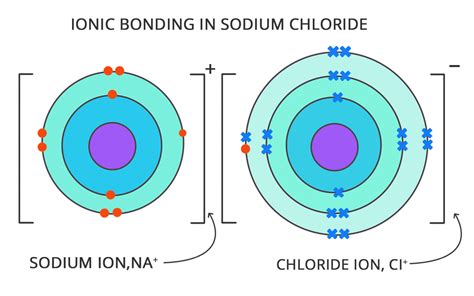
Table of Contents
How Does an Ion Differ from an Atom? A Comprehensive Guide
Understanding the fundamental building blocks of matter is crucial to comprehending chemistry and physics. At the heart of it all lies the atom, the smallest unit of an element that retains its chemical properties. However, atoms are not always content to exist in isolation. They can gain or lose electrons, transforming into charged particles known as ions. This article delves deep into the differences between atoms and ions, exploring their structures, properties, and behaviors.
The Atom: A Neutral Entity
An atom is the basic unit of a chemical element. It's a tiny, incredibly complex structure consisting primarily of three subatomic particles:
-
Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity. For example, all hydrogen atoms have one proton, all helium atoms have two, and so on.
-
Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons in an atom's nucleus can vary, leading to isotopes of the same element. Isotopes have the same number of protons but a different number of neutrons.
-
Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons in a neutral atom is equal to the number of protons, resulting in a net charge of zero. The arrangement of electrons in these shells determines the atom's chemical behavior and reactivity.
The atom's overall structure is primarily defined by the strong nuclear force holding the protons and neutrons together in the nucleus, while the electrostatic force governs the attraction between the positively charged nucleus and the negatively charged electrons.
Atomic Structure and Electron Configuration
Understanding electron configuration is key to grasping the difference between atoms and ions. Electrons occupy specific energy levels or shells, each capable of holding a limited number of electrons. The outermost shell, known as the valence shell, plays a crucial role in determining an atom's reactivity. Atoms tend to achieve stability by having a full valence shell, typically containing eight electrons (the octet rule, with some exceptions).
Atoms with incomplete valence shells are highly reactive, readily interacting with other atoms to gain, lose, or share electrons and achieve stability. This drive for stability is the fundamental force behind chemical bonding and the formation of molecules and compounds.
Ions: Charged Particles
Unlike neutral atoms, ions carry a net electrical charge. This charge arises from an imbalance in the number of protons and electrons. There are two main types of ions:
-
Cations: Positively charged ions formed when an atom loses one or more electrons. This loss of electrons leaves the atom with more protons than electrons, resulting in a positive charge. Metals generally form cations because they tend to lose electrons relatively easily. For instance, a sodium atom (Na) can lose one electron to become a sodium cation (Na⁺).
-
Anions: Negatively charged ions formed when an atom gains one or more electrons. This gain of electrons results in more electrons than protons, leading to a negative charge. Nonmetals often form anions because they readily accept electrons to complete their valence shells. For example, a chlorine atom (Cl) can gain one electron to become a chloride anion (Cl⁻).
Ion Formation and the Octet Rule
The formation of ions is often driven by the octet rule, a principle stating that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell. By losing or gaining electrons, atoms can achieve a more stable electron configuration, similar to that of a noble gas. This stable configuration minimizes the atom's energy, making the ion formation energetically favorable.
However, it's crucial to note that the octet rule is not universally applicable. Some atoms, particularly those in the transition metals, can have more than eight electrons in their valence shells.
Key Differences Between Atoms and Ions
The fundamental distinction between an atom and an ion lies in their electrical charge:
| Feature | Atom | Ion |
|---|---|---|
| Charge | Neutral (0) | Positive (cation) or negative (anion) |
| Electron Number | Equal to proton number | Unequal to proton number |
| Reactivity | Varies, often determined by valence electrons | Generally higher than neutral atoms |
| Formation | Naturally occurring | Formed by gaining or losing electrons |
| Stability | Varies, depends on electron configuration | Generally more stable than corresponding atoms (when following octet rule) |
The Significance of Ions in Chemistry and Biology
Ions play pivotal roles in a wide range of chemical and biological processes:
Chemical Reactions:
Ions are the fundamental building blocks of ionic compounds, where electrostatic forces hold oppositely charged ions together. Table salt (NaCl), for example, is composed of sodium cations (Na⁺) and chloride anions (Cl⁻) held together by ionic bonds. Ions also participate in numerous chemical reactions, such as acid-base reactions and redox reactions (reduction-oxidation reactions involving electron transfer).
Biological Systems:
Ions are essential for the proper functioning of living organisms. For example:
-
Sodium (Na⁺) and Potassium (K⁺) ions: These are crucial for nerve impulse transmission and muscle contraction. The movement of these ions across cell membranes generates electrical signals that enable communication within the body.
-
Calcium (Ca²⁺) ions: Essential for bone formation, muscle contraction, and blood clotting.
-
Chloride (Cl⁻) ions: Involved in maintaining fluid balance and regulating nerve impulses.
-
Magnesium (Mg²⁺) ions: A cofactor for many enzymes and plays a crucial role in various metabolic processes.
-
Phosphate (PO₄³⁻) ions: Essential components of DNA, RNA, and ATP (adenosine triphosphate), the primary energy currency of cells.
Identifying Ions: Nomenclature and Notation
Ions are identified using their chemical symbols with superscripts indicating their charge. For example:
- Na⁺ (sodium cation) indicates a sodium atom that has lost one electron.
- Ca²⁺ (calcium cation) indicates a calcium atom that has lost two electrons.
- Cl⁻ (chloride anion) indicates a chlorine atom that has gained one electron.
- O²⁻ (oxide anion) indicates an oxygen atom that has gained two electrons.
The naming conventions for ions usually follow these rules:
-
Cations: Retain the name of the element. For example, Na⁺ is sodium ion, Ca²⁺ is calcium ion, and Fe³⁺ is iron(III) ion (Roman numerals indicate the charge when the element has multiple possible ionic states).
-
Anions: The name typically ends in "-ide" for monatomic anions (ions formed from a single atom). For example, Cl⁻ is chloride ion, O²⁻ is oxide ion, and S²⁻ is sulfide ion. Polyatomic anions (ions containing multiple atoms) have more complex naming conventions.
Conclusion
The difference between atoms and ions is fundamentally about their electrical charge. Atoms, in their neutral state, have an equal number of protons and electrons. Ions, on the other hand, possess a net positive or negative charge due to an imbalance in the number of protons and electrons, acquired through the gain or loss of electrons. This seemingly simple difference has profound consequences, impacting the chemical and physical properties of these particles, and ultimately shaping the world around us, from the formation of simple compounds to the complex processes that sustain life itself. Understanding this distinction is key to unlocking the complexities of chemistry and biology.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 18 And 30
Apr 02, 2025
-
Miles Per Hour To Meters Per Minute
Apr 02, 2025
-
Has A Definite Volume And Shape
Apr 02, 2025
-
What Is 3 Out Of 25 As A Percentage
Apr 02, 2025
-
Distance From Earth To Mars In Light Years
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Does An Ion Differ From An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
