How Do You Find The Mass Of Liquid
listenit
Mar 21, 2025 · 6 min read
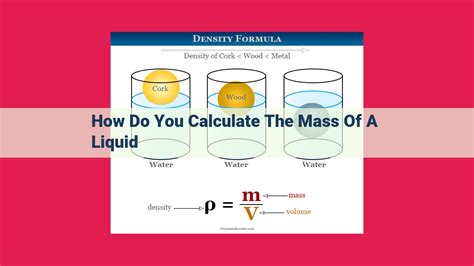
Table of Contents
- How Do You Find The Mass Of Liquid
- Table of Contents
- How Do You Find the Mass of a Liquid? A Comprehensive Guide
- Understanding Mass and its Relationship to Liquids
- Methods for Determining the Mass of a Liquid
- 1. Direct Measurement Using a Scale
- 2. Using Density and Volume: A Precise Approach
- 3. Archimedes' Principle and Buoyancy: An Indirect Method
- Sources of Error and How to Minimize Them
- Choosing the Right Method: A Practical Guide
- Conclusion: Mastering Liquid Mass Determination
- Latest Posts
- Latest Posts
- Related Post
How Do You Find the Mass of a Liquid? A Comprehensive Guide
Determining the mass of a liquid might seem straightforward, but the method you choose depends heavily on what tools you have available and the level of accuracy required. This comprehensive guide explores various techniques, from simple measurements using a scale to more advanced methods involving density and volume calculations. We'll also delve into the importance of accuracy and the potential sources of error in each method.
Understanding Mass and its Relationship to Liquids
Before diving into the methods, let's clarify the fundamental concepts. Mass is the amount of matter in an object, often expressed in grams (g) or kilograms (kg). Unlike weight, which is affected by gravity, mass remains constant regardless of location. Liquids, being a state of matter, possess mass just like solids. The challenge lies in accurately measuring this mass due to the liquid's ability to conform to the shape of its container.
Methods for Determining the Mass of a Liquid
Several methods can be employed to accurately determine the mass of a liquid. The best approach depends on the resources available and the precision needed.
1. Direct Measurement Using a Scale
This is the most straightforward method, assuming you have a suitable scale.
-
Procedure:
- Tare the scale: Place an empty container (beaker, flask, etc.) on the scale and zero it out. This ensures that the scale only measures the mass of the liquid, not the container.
- Add the liquid: Carefully pour the liquid into the tared container.
- Record the mass: The scale will display the mass of the liquid. Record this value carefully, including the units (usually grams or kilograms).
-
Accuracy: The accuracy depends on the sensitivity and calibration of the scale. Analytical balances provide highly accurate measurements, while simpler kitchen scales offer less precision.
-
Advantages: Simple, quick, and generally provides relatively accurate results.
-
Disadvantages: Requires a scale, can be prone to spills, and may not be suitable for very small or very large volumes of liquid.
2. Using Density and Volume: A Precise Approach
This method requires knowledge of the liquid's density and accurate measurement of its volume. It's particularly useful when a scale isn't readily available or when extremely precise measurements are required.
-
Procedure:
- Determine the volume: Use a graduated cylinder, pipette, burette, or other volumetric glassware to accurately measure the volume of the liquid. Record the volume with appropriate units (usually milliliters or liters). Ensure accurate reading by considering the meniscus.
- Find the density: Look up the density of the liquid in a reference table or chemical handbook. The density of a substance is typically given in units of g/mL or kg/L. The density can also be experimentally determined using a pycnometer, although this method introduces additional complexities and potential sources of error.
- Calculate the mass: Use the formula: Mass = Density x Volume
-
Accuracy: The accuracy relies heavily on the accuracy of both the volume and density measurements. Errors in either measurement will directly impact the calculated mass.
-
Advantages: Doesn't require a scale, useful for situations where a scale is unavailable or impractical, allows for high precision if accurate volume measurements and known density are available.
-
Disadvantages: Requires knowledge of the liquid's density, potentially needing a density measuring apparatus, accuracy is entirely dependent on the accuracy of the volume and density measurements. This method is not suitable for liquids with unknown or variable densities.
3. Archimedes' Principle and Buoyancy: An Indirect Method
Archimedes' principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. This principle can be utilized to indirectly determine the mass of a liquid.
-
Procedure:
- Submerge a known mass: Submerge an object of known mass (e.g., a metal weight) in the liquid.
- Measure the apparent weight loss: Measure the apparent weight loss of the object due to buoyancy. This can be done using a spring balance or a scale. The apparent weight loss is equal to the weight of the liquid displaced.
- Calculate the volume of liquid displaced: Assuming the object's density is known, determine the volume of liquid displaced using the object's apparent weight loss and density. (Remember that Weight = Mass * Gravity, where Gravity is usually approximately 9.8 m/s²)
- Determine the density of the liquid: This step assumes that you either know or have a method of measuring the density of the object.
- Calculate the mass of the liquid: Use the formula: Mass = Density of liquid x Volume of liquid displaced.
-
Accuracy: The accuracy depends on the accuracy of the mass of the submerged object, the accuracy of the measurement of the apparent weight loss, and the accuracy of the object's density.
-
Advantages: An alternative method when direct measurement isn't feasible, provides a measure of liquid's density as a byproduct.
-
Disadvantages: More complex than direct measurement, requires accurate measurements of weight loss and understanding of buoyant forces. Errors accumulate easily, therefore requiring skillful execution and potentially multiple measurements for increased accuracy.
Sources of Error and How to Minimize Them
Regardless of the method used, several factors can contribute to errors in mass determination.
-
Measurement Errors: Inaccurate readings from scales or volumetric glassware are common sources of error. Always use calibrated equipment and employ proper measuring techniques (e.g., reading the meniscus at eye level).
-
Temperature Fluctuations: Temperature affects the density of liquids. Changes in temperature can lead to inaccuracies, particularly when using the density-volume method. Maintain a constant temperature throughout the measurement process.
-
Evaporation: Volatile liquids can evaporate during the measurement process, leading to an underestimation of the mass. Minimize evaporation by working quickly and covering the container whenever possible.
-
Calibration of Instruments: Ensure that all instruments used are properly calibrated. Regular calibration is crucial for maintaining accuracy. For scales, utilize calibration weights to ensure accurate reading and adjust as necessary. Volumetric glassware usually needs checking for calibration marks during manufacturing.
-
Spillage: Spillage is a significant source of error when working with liquids. Exercise caution to minimize the possibility of spillage.
Choosing the Right Method: A Practical Guide
The optimal method for determining the mass of a liquid depends on several factors:
-
Availability of Equipment: If you have a scale, direct measurement is the simplest and often most accurate approach. If a scale isn't available, the density-volume method is a viable alternative, provided the density of the liquid is known.
-
Required Accuracy: For high-precision measurements, the density-volume method, using precisely calibrated volumetric glassware, is generally preferred. However, even then, understanding the potential limitations and sources of error is critical to the accuracy of the final reading.
-
Nature of the Liquid: The volatility of the liquid influences the method choice. Volatile liquids require extra care to minimize evaporation losses.
Conclusion: Mastering Liquid Mass Determination
Accurately determining the mass of a liquid is crucial in various scientific and practical applications. Choosing the appropriate method and understanding potential sources of error are essential for achieving reliable results. Whether you're using a simple scale or employing more sophisticated techniques based on density and volume, careful attention to detail is paramount in ensuring the accuracy of your measurements. By understanding these principles and employing best practices, you can confidently and accurately determine the mass of any liquid.
Latest Posts
Latest Posts
-
Difference Between Electronegativity And Electron Affinity
Mar 28, 2025
-
What Is The Formula For Dinitrogen Pentoxide
Mar 28, 2025
-
Two Or More Atoms Bonded Together
Mar 28, 2025
-
Which Set Of Ordered Pairs Does Not Represent A Function
Mar 28, 2025
-
Is Mg A Solid Liquid Or Gas
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Do You Find The Mass Of Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
