Difference Between Electronegativity And Electron Affinity
listenit
Mar 28, 2025 · 6 min read
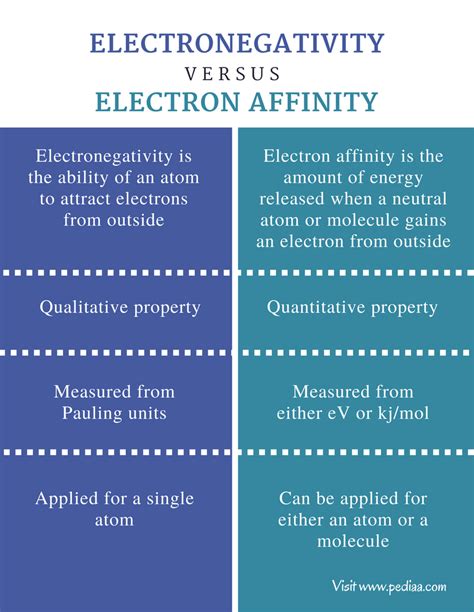
Table of Contents
Electronegativity vs. Electron Affinity: Understanding the Key Differences
Electronegativity and electron affinity are two fundamental concepts in chemistry that describe an atom's tendency to attract electrons. While both relate to an atom's ability to acquire electrons, they differ significantly in their definition, measurement, and application. Understanding these differences is crucial for comprehending chemical bonding, reactivity, and molecular properties. This article will delve into the nuances of electronegativity and electron affinity, highlighting their similarities and, more importantly, their critical distinctions.
What is Electronegativity?
Electronegativity is a measure of an atom's ability to attract electrons within a chemical bond. It's a relative property, meaning it's compared to other atoms within a molecule or compound, not in isolation. Think of it as a tug-of-war for electrons between atoms participating in a bond. The atom with higher electronegativity pulls the shared electrons closer to itself.
Key characteristics of electronegativity:
- Relative property: It's a comparison between atoms in a bond, not an absolute measure of electron attraction.
- Depends on the bonding environment: The electronegativity of an atom can vary slightly depending on the other atoms it's bonded to.
- Influenced by atomic structure: Factors such as nuclear charge, atomic radius, and shielding effect influence electronegativity.
Several scales exist to quantify electronegativity, the most common being the Pauling scale. This scale assigns fluorine (the most electronegative element) a value of 4.0, and other elements are assigned values relative to this. Elements with higher electronegativity values are more likely to attract electrons in a bond, leading to polar or ionic bonds.
Factors Affecting Electronegativity
Several factors contribute to an atom's electronegativity:
- Nuclear Charge: A higher nuclear charge (more protons) exerts a stronger pull on electrons, increasing electronegativity.
- Atomic Radius: Smaller atoms have a shorter distance between the nucleus and the valence electrons, resulting in stronger attraction and higher electronegativity.
- Shielding Effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge experienced by valence electrons, lowering electronegativity.
What is Electron Affinity?
Electron affinity, on the other hand, is the energy change that occurs when a neutral atom in the gaseous phase gains an electron to form a negative ion (anion). It's an absolute measure, reflecting the energy released or absorbed during this process. A positive electron affinity indicates that energy is released (an exothermic process), while a negative electron affinity signifies that energy is absorbed (an endothermic process).
Key characteristics of electron affinity:
- Absolute property: It's a measure of the energy change associated with gaining an electron, not a comparison between atoms.
- Measured in gaseous phase: The atom must be in the gaseous state to ensure accurate measurement, avoiding interactions with other atoms or molecules.
- Reflects stability of anion: A high positive electron affinity suggests the formed anion is relatively stable.
Factors Affecting Electron Affinity
Similar to electronegativity, several factors influence an atom's electron affinity:
- Nuclear Charge: Higher nuclear charge generally leads to a more negative (or more exothermic) electron affinity, as the added electron is strongly attracted to the nucleus.
- Atomic Radius: Smaller atoms experience a stronger attraction for the added electron, resulting in a more negative electron affinity.
- Electron Shielding: Similar to electronegativity, shielding effects reduce the effective nuclear charge, leading to a less negative (or less exothermic) electron affinity.
- Electron Configuration: Half-filled and fully filled subshells are particularly stable. Atoms with these configurations will have relatively low electron affinities. Adding an electron disrupts this stability, requiring energy input (positive electron affinity).
Key Differences Between Electronegativity and Electron Affinity
While both concepts relate to an atom's attraction to electrons, several key differences set them apart:
| Feature | Electronegativity | Electron Affinity |
|---|---|---|
| Definition | Ability to attract electrons within a chemical bond | Energy change upon gaining an electron (gaseous phase) |
| Nature | Relative property | Absolute property |
| Measurement | Scale (e.g., Pauling scale) | Energy change (kJ/mol) |
| Phase | Not limited to gas phase; applies to bonded atoms | Measured in the gaseous phase |
| Context | Within a chemical bond | Isolated atom gaining an electron |
| Application | Predicting bond polarity, bond type | Predicting anion formation, reactivity |
Comparing Electronegativity and Electron Affinity Trends in the Periodic Table
Both electronegativity and electron affinity show trends across the periodic table, although the trends are not perfectly parallel.
Electronegativity:
- Increases across a period: Moving left to right, the nuclear charge increases while the atomic radius decreases, leading to higher electronegativity.
- Decreases down a group: Increasing atomic radius and increased shielding down a group counteract the increased nuclear charge, resulting in lower electronegativity.
Electron Affinity:
- Generally increases across a period: Similar to electronegativity, increased nuclear charge and decreased atomic radius contribute to a more negative (more exothermic) electron affinity. However, there are exceptions due to electron configuration effects (e.g., the relatively low electron affinity of nitrogen and oxygen compared to other elements in the same period).
- Generally decreases down a group: Increasing atomic radius and shielding lead to a less negative (less exothermic) electron affinity as one moves down a group. Again, there are exceptions based on electron configurations and the relative stability of the anions formed.
Applications of Electronegativity and Electron Affinity
Both electronegativity and electron affinity are essential tools in various areas of chemistry:
Electronegativity:
- Predicting bond polarity: The difference in electronegativity between two atoms determines the polarity of the bond formed. A large difference leads to a polar covalent or even an ionic bond, while a small difference results in a nonpolar covalent bond.
- Understanding molecular properties: Electronegativity differences influence the molecular dipole moment, boiling point, and solubility of compounds.
- Predicting reaction mechanisms: Electronegativity helps in understanding which atoms are more likely to act as nucleophiles or electrophiles in a chemical reaction.
Electron Affinity:
- Predicting anion formation: Electron affinity helps determine the likelihood of an atom forming a stable anion. Atoms with high positive electron affinities readily form stable anions.
- Understanding redox reactions: Electron affinity plays a role in understanding the tendency of an atom to gain or lose electrons in redox reactions. Elements with high electron affinities are good oxidizing agents.
- Material science: Electron affinity is critical in designing materials with specific electronic properties, including semiconductors and superconductors.
Conclusion
While both electronegativity and electron affinity are related to an atom's attraction towards electrons, they represent distinct concepts with different definitions, measurements, and applications. Electronegativity compares the electron-attracting ability of atoms within a bond, while electron affinity measures the energy change when a gaseous atom gains an electron. Understanding these differences is crucial for interpreting chemical behaviour and predicting the properties of molecules and materials. Both concepts are indispensable tools for chemists and materials scientists working to uncover the secrets of the atomic world. By understanding these concepts in detail, you'll be better equipped to explain chemical bonding, reactivity, and a multitude of other fundamental chemical phenomena.
Latest Posts
Latest Posts
-
Balanced Equation Of Magnesium And Hydrochloric Acid
Mar 31, 2025
-
Lowest Common Multiple Of 25 And 30
Mar 31, 2025
-
Whats The Square Root Of 576
Mar 31, 2025
-
What Is 1 And 4 5 As A Decimal
Mar 31, 2025
-
4 5 To The Power Of 2
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Electronegativity And Electron Affinity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
