How Are Osmosis And Diffusion Alike
listenit
Mar 20, 2025 · 5 min read
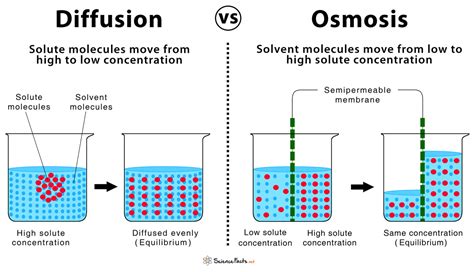
Table of Contents
- How Are Osmosis And Diffusion Alike
- Table of Contents
- How Are Osmosis and Diffusion Alike? Exploring the Similarities in Passive Transport
- The Core Similarity: Passive Transport Mechanisms
- Random Molecular Motion: The Driving Force
- Movement Down the Concentration Gradient
- Diffusion: The General Movement of Molecules
- Factors Affecting Diffusion Rate
- Osmosis: The Diffusion of Water Across a Selectively Permeable Membrane
- Water Potential: The Driving Force in Osmosis
- Osmotic Pressure: A Consequence of Osmosis
- Illustrative Examples: Osmosis and Diffusion in Action
- Distinguishing Features: Where Osmosis and Diffusion Diverge
- Conclusion: Two Sides of the Same Coin
- Latest Posts
- Latest Posts
- Related Post
How Are Osmosis and Diffusion Alike? Exploring the Similarities in Passive Transport
Osmosis and diffusion are fundamental processes in biology, crucial for the transport of substances across cell membranes and within living organisms. While often discussed separately, understanding their similarities is key to grasping their collective importance in maintaining life. This article delves into the shared characteristics of osmosis and diffusion, highlighting their mechanisms, driving forces, and overall significance in biological systems.
The Core Similarity: Passive Transport Mechanisms
At their heart, both osmosis and diffusion are types of passive transport. This means they don't require energy input from the cell to occur. Instead, they rely on the inherent kinetic energy of molecules, their natural tendency to move and spread out. This contrasts with active transport, which utilizes cellular energy (ATP) to move molecules against their concentration gradients. This fundamental similarity is the cornerstone of understanding their relationship.
Random Molecular Motion: The Driving Force
The driving force behind both processes is the random movement of molecules. Molecules are constantly in motion, colliding with each other and their surroundings. This ceaseless motion, also known as Brownian motion, is the engine that propels both diffusion and osmosis. The higher the temperature, the faster the molecules move, and consequently, the faster the rate of both processes.
Movement Down the Concentration Gradient
Another key similarity is the direction of movement. Both osmosis and diffusion involve the net movement of molecules from a region of higher concentration to a region of lower concentration. This is often referred to as moving "down" the concentration gradient. The molecules are essentially spreading out, aiming for a state of equilibrium where they are evenly distributed throughout the available space. This principle of moving down the concentration gradient is a unifying characteristic that defines both processes as passive transport mechanisms.
Diffusion: The General Movement of Molecules
Diffusion is a broader term that describes the net movement of any type of molecule from an area of high concentration to an area of low concentration. This can occur in gases, liquids, or across semi-permeable membranes. Imagine spraying perfume in a room – the perfume molecules will diffuse outwards, eventually filling the entire room. This is a classic example of diffusion.
Factors Affecting Diffusion Rate
Several factors influence the rate of diffusion:
-
Concentration Gradient: A steeper concentration gradient (a larger difference in concentration between two areas) results in faster diffusion. The greater the difference, the stronger the driving force for movement.
-
Temperature: Higher temperatures lead to faster molecular movement and thus, faster diffusion.
-
Mass of the Molecules: Lighter molecules diffuse faster than heavier ones because they move more readily.
-
Surface Area: A larger surface area allows for more molecules to cross the boundary simultaneously, leading to increased diffusion rate.
-
Distance: The shorter the distance molecules need to travel, the faster the diffusion.
Osmosis: The Diffusion of Water Across a Selectively Permeable Membrane
Osmosis is a specialized type of diffusion that specifically focuses on the movement of water molecules across a selectively permeable membrane. A selectively permeable membrane allows some substances to pass through while restricting others. Cell membranes are prime examples of selectively permeable membranes.
Water Potential: The Driving Force in Osmosis
In osmosis, the driving force is the difference in water potential. Water potential is a measure of the tendency of water to move from one area to another. It's influenced by factors like the concentration of solutes (dissolved substances) and pressure. Water moves from areas of high water potential (low solute concentration) to areas of low water potential (high solute concentration). Essentially, water moves to dilute the more concentrated solution.
Osmotic Pressure: A Consequence of Osmosis
As water moves across the membrane during osmosis, it can create pressure. This is known as osmotic pressure. The magnitude of osmotic pressure depends on the concentration difference across the membrane. A larger concentration difference will result in higher osmotic pressure.
Illustrative Examples: Osmosis and Diffusion in Action
Let's examine some examples to solidify our understanding of these similar processes:
-
Oxygen uptake in the lungs: Oxygen diffuses from the alveoli (air sacs in the lungs), where its concentration is high, into the blood capillaries, where its concentration is low. This is simple diffusion of a gas.
-
Nutrient absorption in the small intestine: Nutrients like glucose and amino acids diffuse from the lumen (the inside) of the small intestine into the bloodstream. This is facilitated diffusion, a type of diffusion aided by transport proteins.
-
Water uptake by plant roots: Water moves from the soil (high water potential) into the roots of plants (low water potential) through osmosis. This is crucial for plant growth and survival.
-
Water reabsorption in the kidneys: Water moves by osmosis from the filtrate in the kidneys back into the bloodstream, regulating the body's water balance.
Distinguishing Features: Where Osmosis and Diffusion Diverge
While osmosis and diffusion share many similarities, it's important to highlight their key differences:
| Feature | Diffusion | Osmosis |
|---|---|---|
| Substance | Any molecule | Water only |
| Membrane | May or may not involve a membrane | Always involves a selectively permeable membrane |
| Driving Force | Concentration gradient | Water potential |
Conclusion: Two Sides of the Same Coin
Osmosis and diffusion are both passive transport processes driven by the inherent kinetic energy of molecules and their tendency to move down concentration gradients. While osmosis is a specialized form of diffusion focusing solely on water movement across a selectively permeable membrane, they are fundamentally linked by their reliance on random molecular motion and the pursuit of equilibrium. Understanding their similarities and differences is essential for a complete comprehension of how substances move within and between cells, forming the basis of many vital biological functions. The efficient and coordinated function of these processes is crucial for maintaining homeostasis and supporting life itself.
Latest Posts
Latest Posts
-
How To Find Equation Of Secant Line
Mar 22, 2025
-
How Many Degrees Fahrenheit Is 1 Degree Celsius
Mar 22, 2025
-
Sodium Hydroxide And Sulfuric Acid Reaction
Mar 22, 2025
-
How Many Millimeters Is 6 Cm
Mar 22, 2025
-
How Many S Orbitals Can Be In An Energy Level
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Are Osmosis And Diffusion Alike . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
