Sodium Hydroxide And Sulfuric Acid Reaction
listenit
Mar 22, 2025 · 5 min read
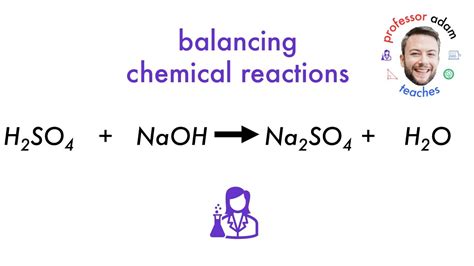
Table of Contents
The Exothermic Reaction Between Sodium Hydroxide and Sulfuric Acid: A Deep Dive
The reaction between sodium hydroxide (NaOH), a strong base, and sulfuric acid (H₂SO₄), a strong acid, is a classic example of a neutralization reaction. It's a highly exothermic process, meaning it releases a significant amount of heat, and is frequently used in various industrial and laboratory settings. Understanding the intricacies of this reaction, including its stoichiometry, energetics, and safety precautions, is crucial for anyone working with these chemicals. This comprehensive article will explore all aspects of this important chemical reaction.
Understanding the Reactants: Sodium Hydroxide and Sulfuric Acid
Before diving into the reaction itself, let's examine the properties of the individual reactants:
Sodium Hydroxide (NaOH)
Sodium hydroxide, also known as caustic soda or lye, is a strong, highly alkaline inorganic compound. It's a white crystalline solid at room temperature, readily soluble in water, and forms strongly alkaline solutions. Its strong basicity stems from its complete dissociation in water, yielding sodium (Na⁺) and hydroxide (OH⁻) ions. This complete dissociation is key to its reactivity in neutralization reactions.
Key Properties of NaOH:
- Strong base: Completely dissociates in water, releasing a high concentration of hydroxide ions.
- Highly corrosive: Can cause severe burns to skin and eyes.
- Hygroscopic: Absorbs moisture from the air.
- Used in: Soap making, paper production, drain cleaners, and various industrial processes.
Sulfuric Acid (H₂SO₄)
Sulfuric acid is a strong, highly corrosive mineral acid. It's a viscous, colorless liquid at room temperature and is miscible with water in all proportions. Similar to NaOH, its strength lies in its complete dissociation in water, albeit in two steps:
H₂SO₄ → H⁺ + HSO₄⁻ HSO₄⁻ ↔ H⁺ + SO₄²⁻
The first dissociation is essentially complete, while the second is only partially complete. Despite this, sulfuric acid still provides a high concentration of hydrogen ions (H⁺), making it a potent acid.
Key Properties of H₂SO₄:
- Strong acid: Produces a high concentration of hydrogen ions in aqueous solutions.
- Highly corrosive: Can cause severe burns to skin and eyes.
- Dehydrating agent: Can remove water molecules from other substances.
- Oxidizing agent: Can oxidize certain substances.
- Used in: Fertilizer production, petroleum refining, metal processing, and battery manufacturing.
The Neutralization Reaction: Sodium Hydroxide and Sulfuric Acid
The reaction between sodium hydroxide and sulfuric acid is a classic acid-base neutralization reaction. The hydrogen ions (H⁺) from the sulfuric acid react with the hydroxide ions (OH⁻) from the sodium hydroxide to form water (H₂O). The remaining sodium ions (Na⁺) and sulfate ions (SO₄²⁻) combine to form sodium sulfate (Na₂SO₄), a salt.
The balanced chemical equation for this reaction is:
2NaOH(aq) + H₂SO₄(aq) → Na₂SO₄(aq) + 2H₂O(l)
This equation shows that two moles of sodium hydroxide react with one mole of sulfuric acid to produce one mole of sodium sulfate and two moles of water. The stoichiometry is crucial for accurately calculating the amounts of reactants needed and the products formed.
The Exothermic Nature of the Reaction
The reaction is highly exothermic, meaning it releases a substantial amount of heat. This heat release is due to the strong attraction between the hydrogen and hydroxide ions, forming stable water molecules. The formation of strong bonds releases energy in the form of heat. The heat released can be significant enough to cause the solution to boil, especially if the reactants are concentrated.
The Formation of Sodium Sulfate
Sodium sulfate (Na₂SO₄) is a soluble salt, meaning it readily dissolves in water. It's a relatively inert compound with a variety of industrial applications, including in the detergent and paper industries. Its formation completes the neutralization process.
Practical Applications of the Reaction
The reaction between sodium hydroxide and sulfuric acid has numerous applications across various industries:
-
Industrial Chemical Production: This reaction is often used as a step in other larger chemical processes. The neutralization reaction can help control pH levels or produce specific compounds.
-
Wastewater Treatment: In wastewater treatment plants, sulfuric acid can be used to neutralize excess alkalinity caused by the presence of sodium hydroxide or other bases.
-
Laboratory Applications: In chemistry labs, this reaction is used for titrations to determine the concentration of either acid or base.
-
pH Control: The reaction can be utilized to precisely adjust the pH of solutions in various industrial processes.
Safety Precautions: Handling Sodium Hydroxide and Sulfuric Acid
Both sodium hydroxide and sulfuric acid are highly corrosive and hazardous substances. Working with these chemicals requires strict adherence to safety protocols:
-
Eye Protection: Always wear safety goggles or a face shield when handling these chemicals.
-
Protective Clothing: Wear appropriate protective clothing, including gloves, lab coats, and closed-toe shoes.
-
Ventilation: Ensure adequate ventilation to prevent inhalation of fumes.
-
Slow Addition: Always add the acid to the base slowly and carefully, stirring constantly, to control the heat generated. Adding the base to the acid can cause violent splashing and boiling.
-
Neutralization: In case of spills, neutralize the spilled chemical with an appropriate counteragent before cleaning it. Never use water to dilute concentrated acid or base – it can cause a violent reaction.
-
First Aid: Have readily available first-aid supplies and know the appropriate first-aid procedures for chemical burns.
Further Considerations: Heat of Neutralization and Enthalpy Change
The heat generated during the neutralization reaction can be quantified using the concept of enthalpy change (ΔH). The enthalpy change represents the change in heat content of the system during the reaction. For the reaction between sodium hydroxide and sulfuric acid, the enthalpy change is highly negative, indicating a large amount of heat is released. This heat release is often used in industrial applications to generate heat or maintain process temperature.
The actual value of the enthalpy change varies depending on the concentrations of the reactants and the temperature of the reaction.
Conclusion: A Powerful and Versatile Reaction
The reaction between sodium hydroxide and sulfuric acid is a powerful, exothermic neutralization reaction with significant industrial and laboratory applications. Understanding the stoichiometry, energetics, and safety precautions associated with this reaction is essential for anyone working with these chemicals. The careful and controlled execution of this reaction is crucial for ensuring both safety and effective results in various contexts, highlighting the importance of understanding its nuances and implications. Always remember to prioritize safety when working with strong acids and bases.
Latest Posts
Latest Posts
-
Use The Indicated Substitution To Evaluate The Integral
May 09, 2025
-
Lipids Are Insoluble In Water Because
May 09, 2025
-
The Density Of Ethanol Is 0 789 G Ml
May 09, 2025
-
Find The Length Of The Polar Curve
May 09, 2025
-
How Are Lactic And Alcoholic Fermentation Similar
May 09, 2025
Related Post
Thank you for visiting our website which covers about Sodium Hydroxide And Sulfuric Acid Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.