How Many S Orbitals Can Be In An Energy Level
listenit
Mar 22, 2025 · 6 min read
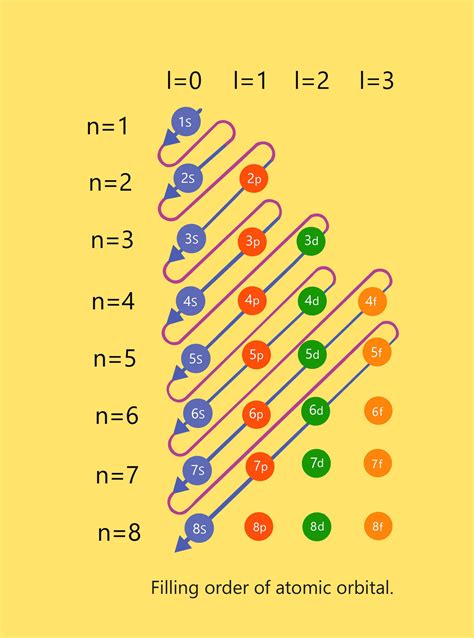
Table of Contents
How Many s Orbitals Can Be in an Energy Level?
Understanding the arrangement of electrons within an atom is fundamental to chemistry. This involves grasping the concepts of energy levels, sublevels, and orbitals. A common question that arises, particularly for students beginning their study of atomic structure, is: how many s orbitals can be found within a given energy level? This article will delve into the intricacies of atomic orbitals, focusing specifically on the s orbitals and their distribution across energy levels. We will explore the underlying principles of quantum mechanics that govern this arrangement and demonstrate the importance of this knowledge in understanding chemical bonding and reactivity.
Understanding Atomic Orbitals
Before we address the central question, let's establish a clear understanding of atomic orbitals. An atomic orbital is a mathematical function that describes the wave-like behavior of an electron in an atom. It doesn't define a precise path the electron follows, but rather a region of space where there's a high probability of finding the electron. These orbitals are characterized by a set of quantum numbers:
-
Principal Quantum Number (n): This number designates the energy level of the electron. It can take on positive integer values (n = 1, 2, 3,...). Higher values of n indicate higher energy levels and greater distances from the nucleus.
-
Azimuthal Quantum Number (l): This number specifies the sublevel or subshell within an energy level. It determines the shape of the orbital and ranges from 0 to n - 1. For example, if n = 2, l can be 0 or 1.
-
Magnetic Quantum Number (ml): This number describes the orientation of the orbital in space. It ranges from -l to +l, including 0.
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can be either +1/2 or -1/2.
s Orbitals: The Spherical Shapes
The s orbital is identified by an azimuthal quantum number (l) of 0. This means that for any given energy level, there can only be one type of s orbital. The defining characteristic of an s orbital is its spherical shape. This means the probability of finding the electron is spherically symmetric around the nucleus. The size of the sphere, however, increases with increasing principal quantum number (n). A 1s orbital is smaller and closer to the nucleus than a 2s orbital, which is smaller than a 3s orbital, and so on.
The Significance of Spherical Symmetry
The spherical symmetry of the s orbital has important implications for chemical bonding. Because the electron density is distributed uniformly around the nucleus, s orbitals can participate effectively in bonding in all directions. This contrasts with orbitals of higher azimuthal quantum numbers (p, d, f), which have more complex shapes and directional properties.
Determining the Number of s Orbitals per Energy Level
Now, let's directly address the main question: how many s orbitals exist within a given energy level? The answer is elegantly simple: only one.
Regardless of the energy level (n), there is always only one s orbital. This is a direct consequence of the azimuthal quantum number (l) being 0 for s orbitals. Since the magnetic quantum number (m<sub>l</sub>) is determined by l, and for l = 0, m<sub>l</sub> can only be 0, there's only one possible orientation for the s orbital.
Example: Energy Level n=3
Consider the third energy level (n = 3). This energy level contains three sublevels:
- s sublevel (l = 0): One s orbital (m<sub>l</sub> = 0)
- p sublevel (l = 1): Three p orbitals (m<sub>l</sub> = -1, 0, +1)
- d sublevel (l = 2): Five d orbitals (m<sub>l</sub> = -2, -1, 0, +1, +2)
Despite the presence of multiple sublevels and orbitals within the n = 3 energy level, there's still only one s orbital. This holds true for all energy levels.
Implications for Electron Configuration
Understanding the number of s orbitals per energy level is crucial for determining the electron configuration of atoms. The electron configuration describes the arrangement of electrons within an atom's orbitals. The Aufbau principle, Hund's rule, and the Pauli exclusion principle are used to predict electron configurations. The Pauli exclusion principle, in particular, states that no two electrons within an atom can have the same set of four quantum numbers. This means each orbital can hold a maximum of two electrons with opposite spins.
Since there's only one s orbital per energy level, this orbital can hold a maximum of two electrons. This fact is reflected in the electron configurations of various elements. For instance:
- Hydrogen (H): 1s<sup>1</sup> (One electron in the 1s orbital)
- Helium (He): 1s<sup>2</sup> (Two electrons in the 1s orbital)
- Lithium (Li): 1s<sup>2</sup>2s<sup>1</sup> (Two electrons in the 1s orbital, one electron in the 2s orbital)
- Beryllium (Be): 1s<sup>2</sup>2s<sup>2</sup> (Two electrons in the 1s orbital, two electrons in the 2s orbital)
And so on. The filling of s orbitals always precedes the filling of p, d, and f orbitals according to the Aufbau principle.
Beyond the Basics: Radial Nodes
While we've focused on the shape and number of s orbitals, it's also important to briefly discuss radial nodes. These are regions within an orbital where the probability of finding an electron is zero. The number of radial nodes in an s orbital is n - 1, where n is the principal quantum number.
For example:
- 1s orbital (n = 1): 1 - 1 = 0 radial nodes (no nodes)
- 2s orbital (n = 2): 2 - 1 = 1 radial node (one node)
- 3s orbital (n = 3): 3 - 1 = 2 radial nodes (two nodes)
These nodes are spherical surfaces and reflect the complex wave nature of electrons within the atom. The presence of nodes doesn't alter the fact that there's only one s orbital per energy level, but it adds to a more complete description of the electron's spatial distribution.
Conclusion: One s Orbital, Countless Applications
In summary, the number of s orbitals within any given energy level is always one. This fundamental principle is a direct consequence of the quantum numbers that describe atomic orbitals. Understanding this seemingly simple fact is crucial for grasping more complex concepts in chemistry, such as electron configuration, bonding, and molecular geometry. The seemingly simple spherical s orbital plays a critical role in determining the chemical behavior of atoms and molecules, making its study vital for anyone seeking a deeper understanding of the chemical world. The one s orbital per energy level, while seemingly a minor detail, serves as a cornerstone of our understanding of atomic structure and the building blocks of matter. Its consistent presence across all energy levels highlights the elegant simplicity and underlying order within the quantum mechanical description of the atom.
Latest Posts
Latest Posts
-
The Sum Of A Number And 3
May 09, 2025
-
What Is The Name Of The Molecular Compound So3
May 09, 2025
-
What Is 70 F To Celsius
May 09, 2025
-
Write 1 8 As A Decimal Number
May 09, 2025
-
What Is The Greatest Common Factor Of 8 And 18
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many S Orbitals Can Be In An Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.