How Are Elements Related To Compounds
listenit
Mar 22, 2025 · 7 min read
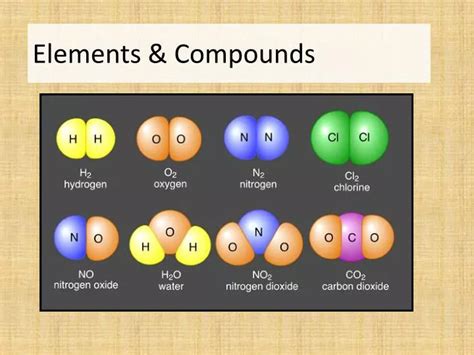
Table of Contents
How Are Elements Related to Compounds? A Deep Dive into Chemical Bonding
The world around us is composed of matter, and matter is made up of elements and compounds. Understanding the relationship between these fundamental building blocks of chemistry is crucial to grasping the complexity of the universe. This article explores the intricate connection between elements and compounds, delving into the process of chemical bonding, different types of compounds, and the properties that emerge from these elemental combinations.
Understanding Elements: The Fundamental Building Blocks
Elements are pure substances that cannot be broken down into simpler substances by chemical means. Each element is defined by its atomic number, which represents the number of protons in the nucleus of its atoms. The periodic table organizes elements based on their atomic number and recurring chemical properties. These elements, ranging from the ubiquitous hydrogen to the rare and radioactive plutonium, serve as the fundamental constituents of all matter.
Key Characteristics of Elements:
- Unique Atomic Structure: Each element possesses a distinct atomic structure characterized by a specific number of protons, neutrons, and electrons. This structure dictates its chemical behavior.
- Defined Properties: Elements exhibit unique physical and chemical properties, such as melting point, boiling point, density, reactivity, and conductivity. These properties are used to identify and distinguish them.
- Indivisible by Chemical Means: Elements cannot be broken down into simpler substances through ordinary chemical reactions. Nuclear reactions, however, can alter the structure of elements.
The Formation of Compounds: Combining Elements
Compounds are pure substances formed when two or more different elements are chemically bonded together in fixed proportions. This chemical bonding involves the interaction of electrons within the atoms of the constituent elements. The properties of a compound are distinctly different from the properties of its constituent elements, a testament to the transformative power of chemical bonding.
The Role of Chemical Bonding:
Chemical bonds are the forces that hold atoms together in compounds. Several types of chemical bonds exist, each influencing the properties of the resulting compound:
-
Ionic Bonds: These bonds form when one or more electrons are transferred from one atom to another. This transfer creates ions – charged particles – that are attracted to each other through electrostatic forces. For example, in sodium chloride (table salt), NaCl, sodium (Na) loses an electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions, which are held together by ionic bonds. Ionic compounds typically have high melting points and are often soluble in water.
-
Covalent Bonds: These bonds involve the sharing of electrons between atoms. Atoms share electrons to achieve a more stable electron configuration, typically resembling a noble gas. Covalent compounds are abundant, encompassing a vast array of organic and inorganic molecules. Water (H₂O) and methane (CH₄) are examples of molecules formed through covalent bonding. Properties of covalent compounds vary widely, depending on the atoms involved and the structure of the molecule.
-
Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and shared among a "sea" of electrons. This allows for high electrical and thermal conductivity and malleability. Metals such as iron (Fe) and copper (Cu) exhibit metallic bonding.
Types of Compounds: Diversity in Chemical Combinations
The combination of elements through diverse bonding mechanisms results in a vast array of compounds with vastly different properties. Compounds are broadly categorized based on their composition and bonding characteristics:
Inorganic Compounds:
Inorganic compounds typically do not contain carbon-hydrogen bonds (with few exceptions like carbonates and cyanides). They encompass a broad range of substances, including:
-
Salts: Formed from the reaction between an acid and a base, salts are ionic compounds that are usually crystalline solids. Examples include sodium chloride (NaCl), potassium nitrate (KNO₃), and calcium carbonate (CaCO₃).
-
Oxides: Compounds containing oxygen and another element. They can be acidic, basic, or amphoteric, depending on the other element. Examples include water (H₂O), carbon dioxide (CO₂), and iron oxide (Fe₂O₃).
-
Acids: Substances that donate protons (H⁺ ions) in solution. They typically react with bases to form salts and water. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
-
Bases: Substances that accept protons (H⁺ ions) in solution. They often react with acids to form salts and water. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂).
Organic Compounds:
Organic compounds predominantly contain carbon and hydrogen, often along with other elements such as oxygen, nitrogen, sulfur, and phosphorus. They form the basis of life and encompass a vast array of molecules, including:
-
Hydrocarbons: Compounds composed solely of carbon and hydrogen. They can be aliphatic (linear or branched chains) or aromatic (containing benzene rings). Examples include methane (CH₄), ethane (C₂H₆), and benzene (C₆H₆).
-
Carbohydrates: Compounds composed of carbon, hydrogen, and oxygen, often in a 1:2:1 ratio. They serve as energy sources and structural components in living organisms. Examples include glucose (C₆H₁₂O₆), sucrose (table sugar), and starch.
-
Lipids: A diverse group of hydrophobic (water-insoluble) compounds, including fats, oils, and waxes. They serve as energy storage, structural components of cell membranes, and hormones.
-
Proteins: Polymers composed of amino acids, which play vital roles in diverse biological processes, including catalysis, structural support, and transport.
-
Nucleic Acids: Polymers of nucleotides that carry genetic information (DNA and RNA).
Properties of Compounds vs. Elements: A Transformative Relationship
The properties of a compound are markedly different from the properties of its constituent elements. This difference arises from the way atoms are bonded together and interact in the compound. For instance:
-
Sodium (Na) is a highly reactive, soft, silvery-white metal, while chlorine (Cl) is a toxic, greenish-yellow gas. However, sodium chloride (NaCl), or table salt, is a non-toxic, crystalline solid that is essential for human life.
-
Hydrogen (H) is a highly flammable gas, and oxygen (O) is a vital gas for respiration. Their combination forms water (H₂O), a liquid essential for life, which is neither flammable nor toxic (in pure form).
This transformation illustrates the significant alteration of properties that occurs when elements combine to form compounds.
The Importance of Stoichiometry: Quantifying the Relationship
Stoichiometry is the study of quantitative relationships between reactants and products in chemical reactions. It’s crucial for understanding the precise ratios in which elements combine to form compounds. Chemical formulas, such as H₂O for water or NaCl for sodium chloride, represent these fixed ratios. For example, the formula H₂O indicates that one molecule of water contains two hydrogen atoms and one oxygen atom. This precise ratio is essential for the chemical and physical properties of water.
Advanced Concepts: Intermolecular Forces and Crystal Structures
Beyond the individual bonds within a compound, intermolecular forces play a significant role in determining the overall properties of the substance. These forces are attractions between molecules, influencing properties like boiling point, melting point, and solubility.
Furthermore, the arrangement of atoms and molecules within a compound, known as its crystal structure, influences its macroscopic properties. Different crystal structures lead to different densities, hardness, and other characteristics. Understanding these aspects adds depth to the understanding of the relationship between elements and compounds.
Conclusion: A Symphony of Elements
The relationship between elements and compounds is a fundamental concept in chemistry. Elements are the fundamental building blocks, and their combination through various chemical bonds gives rise to the immense diversity of compounds that make up our world. Understanding the types of chemical bonds, the properties of different compounds, and the principles of stoichiometry provides a deeper insight into the intricate nature of matter and the remarkable transformations that occur when elements combine. From the simple molecules of water to the complex biomolecules of life, the relationship between elements and compounds remains a central theme in the fascinating field of chemistry.
Latest Posts
Latest Posts
-
2 1 2 As A Improper Fraction
Mar 22, 2025
-
Ground State To Excited State Absorbs Energy
Mar 22, 2025
-
How Many Inches In 6 Meters
Mar 22, 2025
-
How Many Electrons Does Iodine Have
Mar 22, 2025
-
Is Viscosity A Chemical Or Physical Property
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements Related To Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
