Ground State To Excited State Absorbs Energy
listenit
Mar 22, 2025 · 6 min read
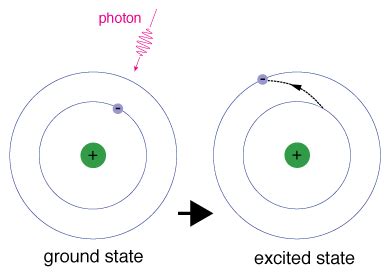
Table of Contents
From Ground State to Excited State: Understanding Energy Absorption in Atoms and Molecules
The world around us is a vibrant tapestry woven from the interactions of light and matter. At the heart of this interaction lies the fundamental process of energy absorption, where atoms and molecules transition from their ground state to an excited state. This seemingly simple event is the driving force behind a multitude of phenomena, from photosynthesis in plants to the vibrant colors of gemstones and the operation of lasers. This comprehensive article delves into the intricacies of this process, exploring the underlying principles, the various mechanisms involved, and its far-reaching consequences.
Understanding Atomic and Molecular Structure: The Foundation of Energy Absorption
Before diving into the absorption process itself, it's crucial to grasp the basic structure of atoms and molecules. Atoms consist of a central nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons occupy specific energy levels or orbitals, dictated by quantum mechanics. The lowest energy level is known as the ground state, representing the most stable configuration of the atom.
Molecules, being collections of atoms bound together, possess a more complex energy structure. Their energy levels are influenced by the interactions between constituent atoms, including bonding and non-bonding interactions. Like atoms, molecules also possess a ground state, representing their most stable configuration.
Quantized Energy Levels: The Key to Absorption
A critical concept in understanding energy absorption is the quantization of energy levels. Electrons in atoms and molecules can only exist in specific, discrete energy levels. They cannot occupy energies between these levels. This is a direct consequence of the wave-particle duality of electrons, as described by quantum mechanics. This means that the energy an electron possesses is not continuous but rather restricted to specific values.
The Role of Photons: The Energy Carriers
The energy required to excite an atom or molecule from its ground state to a higher energy level (excited state) is typically provided by photons – particles of light. Each photon carries a specific amount of energy, directly proportional to its frequency (or inversely proportional to its wavelength). This relationship is expressed by the equation:
E = hν = hc/λ
Where:
- E is the energy of the photon
- h is Planck's constant
- ν is the frequency of the photon
- c is the speed of light
- λ is the wavelength of the photon
The Absorption Process: From Ground to Excited State
Energy absorption occurs when a photon interacts with an atom or molecule. If the energy of the photon (E) precisely matches the energy difference (ΔE) between the ground state and a specific excited state, the photon can be absorbed. This absorption process elevates the electron to the higher energy level, leaving the atom or molecule in an excited state. This is often visualized as an electron "jumping" to a higher energy orbital.
Resonance and Selection Rules: Not All Transitions are Equal
Not all transitions between energy levels are equally probable. The probability of a transition depends on several factors, including the nature of the initial and final states and the polarization of the incident light. These factors are governed by selection rules, which dictate which transitions are allowed and which are forbidden. These rules are crucial in determining the absorption spectrum of a substance – the specific wavelengths of light that a substance absorbs.
The Lifetime of Excited States: A Transient Existence
Excited states are inherently unstable. The electron in the excited state will eventually return to a lower energy level, emitting a photon in the process. The time an electron spends in an excited state is known as its lifetime, and it varies greatly depending on the specific atom or molecule and the energy levels involved. This process of returning to the ground state, accompanied by photon emission, is known as fluorescence or phosphorescence, depending on the mechanism involved.
Mechanisms of Energy Absorption: A Closer Look
The absorption of energy is not a uniform process. Different mechanisms are at play depending on the nature of the interacting system and the type of radiation involved.
Electronic Transitions: Excitation of Electrons
Electronic transitions involve the excitation of electrons from one electronic energy level to another. These transitions are typically induced by ultraviolet (UV) or visible light, leading to changes in the electronic configuration of the atom or molecule. The energy difference between electronic levels is generally large, resulting in the absorption of high-energy photons. These transitions are responsible for the vibrant colors of many materials.
Vibrational Transitions: Molecular Motion
Molecules, in addition to electronic energy levels, possess vibrational energy levels associated with the stretching and bending of chemical bonds. Transitions between vibrational levels are typically induced by infrared (IR) radiation. The energy differences between vibrational levels are smaller than electronic energy levels, leading to the absorption of lower-energy photons. IR spectroscopy is a powerful tool for studying molecular vibrations and identifying functional groups in molecules.
Rotational Transitions: Molecular Rotation
Molecules also possess rotational energy levels associated with their rotation in space. Transitions between rotational levels are typically induced by microwave radiation. The energy differences between rotational levels are the smallest among the three types of transitions discussed here, leading to the absorption of even lower-energy photons. Microwave spectroscopy is used to study molecular rotations and determine molecular structures.
Applications of Energy Absorption: From Spectroscopy to Lasers
The principle of energy absorption from the ground state to an excited state underpins a wide range of applications across diverse scientific and technological fields.
Spectroscopy: Unraveling Molecular Structure
Spectroscopy involves analyzing the absorption or emission of electromagnetic radiation by a substance to determine its composition, structure, and properties. Various spectroscopic techniques exploit different energy transitions (electronic, vibrational, rotational) to obtain detailed information about the sample. Spectroscopy plays a vital role in various fields, including chemistry, physics, biology, and materials science.
Laser Technology: Harnessing Excited States
Lasers (Light Amplification by Stimulated Emission of Radiation) function based on the principle of stimulated emission, which relies on the presence of atoms or molecules in excited states. When a photon interacts with an excited atom or molecule, it can stimulate the emission of an identical photon, leading to amplification of light. Lasers have revolutionized numerous applications, from medical treatments and optical communication to industrial processing and scientific research.
Photosynthesis: Nature's Energy Conversion
Photosynthesis in plants involves the absorption of light energy by chlorophyll molecules. This absorption process elevates electrons to excited states, initiating a chain of reactions that ultimately convert light energy into chemical energy in the form of glucose. Photosynthesis is essential for the sustenance of most life on Earth.
Conclusion: A Fundamental Process with Broad Implications
The transition from the ground state to an excited state upon energy absorption is a fundamental process with profound implications across various disciplines. Understanding the underlying principles and mechanisms of this process is crucial for advancing our knowledge in fields ranging from materials science and chemistry to biology and physics. As we continue to explore the intricacies of light-matter interactions, we can expect further advancements and innovations based on this ubiquitous phenomenon. The ability to manipulate and control energy absorption will undoubtedly continue to shape future technological advancements and our understanding of the natural world.
Latest Posts
Latest Posts
-
Length Of A Line Segment Formula
Mar 23, 2025
-
How To Calculate Mass Of Excess Reactant
Mar 23, 2025
-
X 2 Y 2 Z 2
Mar 23, 2025
-
What Is The Value Of 10
Mar 23, 2025
-
What Is The Difference Between The Purines And The Pyrimidines
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Ground State To Excited State Absorbs Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
