What Is The Difference Between The Purines And The Pyrimidines
listenit
Mar 23, 2025 · 6 min read
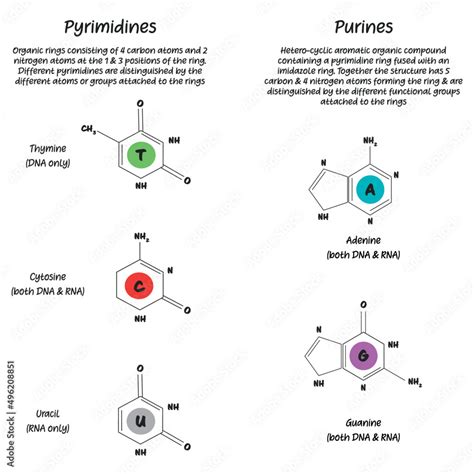
Table of Contents
Purines vs. Pyrimidines: A Deep Dive into the Building Blocks of Nucleic Acids
Nucleic acids, the fundamental molecules of life, are composed of long chains of nucleotides. These nucleotides, in turn, are built from three components: a sugar (ribose or deoxyribose), a phosphate group, and a nitrogenous base. These nitrogenous bases are the stars of our show today, specifically the crucial differences between purines and pyrimidines. Understanding their distinct structures and roles is key to grasping the intricacies of DNA, RNA, and the processes they govern.
Understanding the Basic Structures: Purines and Pyrimidines
The nitrogenous bases are categorized into two families: purines and pyrimidines. The distinction lies in their ring structures. This structural difference has profound consequences for their interactions and functions within nucleic acids.
Purines: The Double-Ringed Structures
Purines are characterized by their double-ring structure: a six-membered ring fused to a five-membered ring. This bicyclic structure makes them larger and more complex than pyrimidines. There are two principal purines found in DNA and RNA:
-
Adenine (A): Adenine is a crucial component of both DNA and RNA. It pairs with thymine (T) in DNA and uracil (U) in RNA through hydrogen bonding. It also plays a vital role in energy transfer as part of adenosine triphosphate (ATP).
-
Guanine (G): Guanine is another essential purine found in both DNA and RNA. It pairs with cytosine (C) in both molecules through a stronger hydrogen bond than the A-T/U pairing.
Pyrimidines: The Single-Ringed Structures
Pyrimidines possess a single six-membered ring structure. Their smaller size compared to purines influences their interactions within the DNA double helix and RNA structures. The major pyrimidines include:
-
Cytosine (C): Cytosine is present in both DNA and RNA and pairs with guanine (G). Its presence in both types of nucleic acids highlights its fundamental role in genetic information.
-
Thymine (T): Thymine is exclusively found in DNA and pairs with adenine (A). Its presence distinguishes DNA from RNA.
-
Uracil (U): Uracil replaces thymine in RNA and also pairs with adenine (A). The absence of a methyl group compared to thymine makes it chemically distinct.
Key Differences Between Purines and Pyrimidines: A Comparative Table
| Feature | Purines | Pyrimidines |
|---|---|---|
| Ring Structure | Double ring (six-membered and five-membered fused) | Single six-membered ring |
| Size | Larger | Smaller |
| Members | Adenine (A), Guanine (G) | Cytosine (C), Thymine (T), Uracil (U) |
| DNA Presence | Adenine (A), Guanine (G) | Cytosine (C), Thymine (T) |
| RNA Presence | Adenine (A), Guanine (G) | Cytosine (C), Uracil (U) |
| Hydrogen Bonds | Guanine forms 3, Adenine forms 2 | Cytosine forms 3, Thymine/Uracil forms 2 |
The Significance of Base Pairing: Chargaff's Rules and Beyond
The specific pairing between purines and pyrimidines – A with T/U and G with C – is dictated by their structural complementarity and the formation of hydrogen bonds. This principle, known as Chargaff's rules, is fundamental to the structure and function of DNA and RNA. The precise number of hydrogen bonds (two for A-T/U and three for G-C) influences the stability of the double helix in DNA and the secondary structures in RNA.
The consistent pairing of one purine with one pyrimidine ensures that the DNA double helix maintains a uniform diameter. If two purines paired together, the helix would bulge; if two pyrimidines paired, it would constrict. This precise arrangement is essential for the accurate replication and transcription of genetic information.
Purine and Pyrimidine Metabolism: Synthesis and Degradation
The synthesis and degradation of purines and pyrimidines are complex metabolic pathways with significant implications for health. De novo synthesis refers to the creation of these bases from simpler molecules, while salvage pathways utilize pre-formed bases or nucleosides to synthesize nucleotides.
Purine Metabolism: A More Complex Pathway
Purine biosynthesis is a more intricate and energy-intensive process compared to pyrimidine biosynthesis. It involves multiple enzymatic steps and requires the contribution of various amino acids, including glycine, aspartate, and glutamine, as well as other molecules like tetrahydrofolate and CO2. The end product is inosine monophosphate (IMP), a precursor to both AMP (adenosine monophosphate) and GMP (guanosine monophosphate).
Purine degradation leads to the formation of uric acid, which is excreted in urine. Disruptions in purine metabolism can lead to conditions like gout, characterized by the accumulation of uric acid crystals in the joints.
Pyrimidine Metabolism: A More Efficient Route
Pyrimidine biosynthesis is comparatively simpler and less energy-demanding. It starts with the formation of carbamoyl phosphate, which subsequently reacts with aspartate to create orotic acid. Orotic acid is then converted to uridine monophosphate (UMP), which serves as a precursor to cytidine triphosphate (CTP) and thymidine triphosphate (TTP).
Pyrimidine degradation is less complex than purine degradation and yields simpler molecules like β-alanine and β-aminoisobutyrate, which can be further metabolized.
Clinical Significance of Purine and Pyrimidine Metabolism
Disruptions in purine and pyrimidine metabolism can lead to various genetic disorders and diseases. These conditions often manifest due to defects in enzymes involved in the synthesis or degradation of these bases.
Some examples include:
-
Lesch-Nyhan syndrome: A rare X-linked recessive disorder caused by a deficiency in hypoxanthine-guanine phosphoribosyltransferase (HGPRT), an enzyme involved in purine salvage. It leads to severe neurological symptoms, including self-mutilating behavior.
-
Orotic aciduria: A rare inherited disorder characterized by the accumulation of orotic acid in the body due to defects in the enzymes involved in pyrimidine biosynthesis. It typically presents with megaloblastic anemia and growth retardation.
-
Gout: As previously mentioned, gout is a metabolic disorder often associated with hyperuricemia, resulting from an increased production or decreased excretion of uric acid, a product of purine catabolism.
Understanding purine and pyrimidine metabolism is essential for diagnosing and treating these disorders.
Purines and Pyrimidines in Research and Therapeutics
Purine and pyrimidine analogs, which are structurally similar to natural bases, have found widespread applications in research and therapeutics. These analogs can inhibit enzymes involved in nucleic acid synthesis, interfering with DNA replication and RNA transcription, thereby impacting cellular processes. They are commonly used as:
-
Chemotherapeutic agents: Many anticancer drugs are purine or pyrimidine analogs. They work by interfering with DNA replication and inhibiting tumor cell growth. Examples include 5-fluorouracil (5-FU), a pyrimidine analog, and 6-mercaptopurine (6-MP), a purine analog.
-
Antiviral agents: Some antiviral drugs target viral DNA or RNA synthesis using purine or pyrimidine analogs. These drugs selectively inhibit viral replication without significantly affecting the host cell.
-
Immunosuppressants: Certain purine and pyrimidine analogs are used as immunosuppressants to prevent organ rejection after transplantation or to treat autoimmune diseases. Azathioprine, a purine analog, is a common example.
Conclusion: The Vital Roles of Purines and Pyrimidines
Purines and pyrimidines are the fundamental building blocks of DNA and RNA, the molecules that carry genetic information and govern cellular processes. Their distinct structures, base pairing specificities, and metabolic pathways are crucial for the integrity and function of genetic material. Furthermore, their importance extends to medicine and research, where purine and pyrimidine analogs are employed as effective therapeutic agents. A thorough understanding of these vital molecules is essential for advancing our knowledge of biology and developing novel treatments for various diseases.
Latest Posts
Latest Posts
-
The Basic Unit Of Mass In The Metric System
Mar 25, 2025
-
Lowest Common Multiple Of 5 And 10
Mar 25, 2025
-
Where Is Absolute Value On Ti 84
Mar 25, 2025
-
Which Element Has A Higher Ionization Energy
Mar 25, 2025
-
1 8 Of A Yard Is How Many Inches
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between The Purines And The Pyrimidines . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
