Groups 3 12 On The Periodic Table
listenit
Mar 30, 2025 · 7 min read
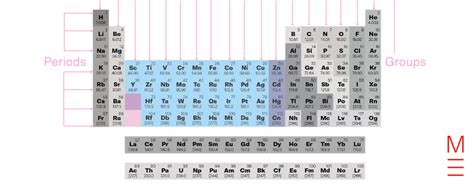
Table of Contents
Groups 3-12: The Transition Metals - A Deep Dive into their Properties and Applications
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Within this organized system, groups 3-12 stand out as the transition metals, a fascinating collection of elements sharing distinctive characteristics that lead to a wide range of applications in modern technology and everyday life. This in-depth exploration will delve into the unique properties of these elements, their chemical behavior, and their crucial roles in various industries.
Defining the Transition Metals: A Unique Set of Characteristics
Transition metals, also known as d-block elements, occupy the central region of the periodic table, bridging the gap between the highly reactive alkali and alkaline earth metals (Groups 1 and 2) and the less reactive main group elements (Groups 13-18). Their defining feature lies in the partially filled d orbitals in their atoms or ions. This incomplete d-orbital configuration is responsible for many of their characteristic properties.
Key Properties of Groups 3-12 Elements:
-
Variable Oxidation States: Unlike many main group elements, transition metals can exhibit multiple oxidation states. This means they can lose different numbers of electrons to form ions with varying charges. For example, iron (Fe) commonly exists in +2 (ferrous) and +3 (ferric) oxidation states. This versatility allows them to participate in a wide array of chemical reactions.
-
Complex Ion Formation: Transition metals readily form complex ions, which involve the metal ion bonded to one or more ligands (molecules or ions). The formation of these complexes often results in vibrant colors, a characteristic feature of many transition metal compounds. The color arises from the electronic transitions within the d orbitals when light interacts with the complex.
-
Catalytic Activity: Many transition metals and their compounds act as excellent catalysts, speeding up chemical reactions without being consumed themselves. This property is crucial in many industrial processes, including the production of plastics, fertilizers, and pharmaceuticals. The ability to exist in multiple oxidation states is key to their catalytic behavior.
-
Magnetic Properties: Several transition metals and their compounds exhibit magnetic properties, such as ferromagnetism (like iron), paramagnetism (like copper), or diamagnetism. These magnetic properties arise from the unpaired electrons in their d orbitals.
-
High Melting and Boiling Points: Transition metals generally possess high melting and boiling points compared to main group elements. This is due to the strong metallic bonding resulting from the delocalized electrons in their d orbitals.
-
Conductivity: Transition metals are excellent conductors of electricity and heat, owing to the mobile electrons in their metallic structure. This high conductivity makes them invaluable in electrical wiring and other applications requiring efficient electron transport.
Exploring Individual Groups Within 3-12: A Closer Look
While sharing common characteristics, each group within the transition metals displays unique properties influencing their individual applications.
Group 3: Scandium, Yttrium, Lanthanum, and Actinium
Group 3 elements are often considered the starting point of the transition series. They are relatively rare and their properties are less pronounced compared to elements in other transition metal groups. Scandium finds limited use in high-intensity lighting and certain alloys. Yttrium is crucial in producing high-temperature superconductors and is also found in lasers. Lanthanum is employed in certain catalysts and in the production of specialized glasses. Actinium, being radioactive, finds limited practical applications.
Group 4: Titanium, Zirconium, and Hafnium
Group 4 elements are known for their strength, high melting points, and corrosion resistance. Titanium is a lightweight, strong metal with excellent corrosion resistance, making it ideal for aerospace applications, biomedical implants, and sporting equipment. Zirconium is used in nuclear reactors due to its low neutron absorption cross-section. Hafnium is employed in control rods for nuclear reactors, taking advantage of its high neutron absorption capacity.
Group 5: Vanadium, Niobium, and Tantalum
Vanadium, niobium, and tantalum have high melting points and are utilized in various high-temperature applications. Vanadium is added to steel to enhance its strength and toughness. Niobium is used in superconducting magnets and high-strength alloys. Tantalum, known for its corrosion resistance, finds application in electronics and surgical implants.
Group 6: Chromium, Molybdenum, and Tungsten
This group features elements known for their high hardness and resistance to corrosion. Chromium is extensively used as a protective coating on steel (chroming), enhancing its resistance to rust. Molybdenum is used in high-strength alloys and as a catalyst. Tungsten boasts an extremely high melting point, making it suitable for filaments in light bulbs and high-speed cutting tools.
Group 7: Manganese, Technetium, and Rhenium
Manganese is essential in steel production, enhancing its strength and toughness. Technetium, a radioactive element, is used in medical imaging techniques. Rhenium is a high-melting point metal used in high-temperature alloys and catalysts.
Group 8: Iron, Ruthenium, Osmium
Iron, a cornerstone of modern civilization, is a fundamental component of steel and countless other alloys. Ruthenium and osmium are less common but are used in specialized alloys and catalysts.
Group 9: Cobalt, Rhodium, Iridium
Cobalt is used in magnets, alloys, and pigments. Rhodium is employed as a catalyst in automotive catalytic converters. Iridium, known for its resistance to corrosion and high melting point, finds use in spark plugs and specialized crucibles.
Group 10: Nickel, Palladium, Platinum
Nickel is a key component in stainless steel and various alloys. Palladium and platinum are valuable precious metals, highly prized for their catalytic properties. They are used in catalytic converters, jewelry, and chemical processes.
Group 11: Copper, Silver, and Gold
Copper, silver, and gold are well-known for their excellent electrical conductivity and malleability. Copper is used extensively in electrical wiring and plumbing. Silver is utilized in photography and electronics. Gold, a highly inert metal, is used in jewelry, electronics, and dentistry.
Group 12: Zinc, Cadmium, and Mercury
Zinc is a crucial element in galvanization, protecting iron and steel from corrosion. Cadmium is used in certain batteries, although its toxicity is a growing concern. Mercury, a liquid metal at room temperature, has historically been used in thermometers and barometers, but its toxicity has led to its phase-out in many applications.
Applications of Transition Metals: A Diverse Landscape
The unique properties of transition metals make them indispensable in a vast array of applications, impacting various aspects of modern life.
Industrial Applications:
-
Steel Production: Iron, along with various alloying elements like chromium, manganese, vanadium, and nickel, forms the basis of steel, a fundamental material in construction, transportation, and manufacturing.
-
Catalysis: Transition metals and their compounds play a crucial role in numerous industrial catalytic processes, including the Haber-Bosch process for ammonia synthesis, the production of plastics, and petroleum refining.
-
Pigments and Dyes: Many transition metal compounds exhibit vibrant colors, making them valuable pigments and dyes used in paints, textiles, and cosmetics.
-
Alloys: Transition metals are often alloyed with other metals to enhance their properties, resulting in materials with improved strength, hardness, corrosion resistance, or other desirable characteristics.
Technological Applications:
-
Electronics: Transition metals are crucial components in electronics, used in conductors, semiconductors, and magnetic storage devices.
-
Biomedical Applications: Titanium and other transition metals are employed in biomedical implants due to their biocompatibility and corrosion resistance.
-
Energy Applications: Transition metals are integral parts of batteries, fuel cells, and other energy technologies.
-
Automotive Industry: Transition metals play a critical role in catalytic converters used in automobiles to reduce harmful emissions.
Everyday Applications:
-
Coins: Copper, nickel, and other transition metals are commonly used in coinage.
-
Jewelry: Gold, silver, platinum, and palladium are highly prized for their beauty and durability in jewelry.
-
Paints and Coatings: Transition metal-based pigments and coatings protect surfaces from corrosion and enhance their aesthetic appeal.
Conclusion: The Enduring Significance of Transition Metals
The transition metals, encompassing Groups 3-12 on the periodic table, represent a group of elements with remarkable properties and diverse applications. Their unique characteristics, including variable oxidation states, complex ion formation, catalytic activity, and magnetic properties, underpin their importance across various sectors. From the skyscrapers and bridges we build to the electronics we rely on, the role of transition metals in our modern world is undeniable. Further research and development are continuously expanding our understanding of these elements and their potential for future technological advancements. As we continue to explore their properties and interactions, the significance of transition metals will only continue to grow.
Latest Posts
Latest Posts
-
How Many Molecules Of Sulfur Trioxide Are In 78 0 Grams
Apr 01, 2025
-
What Is The Least Common Multiple Of 10 14
Apr 01, 2025
-
What Is 6 To The Power Of 0
Apr 01, 2025
-
What Is The Oxidation State Of S In H2so4
Apr 01, 2025
-
Why Is Water A Liquid At Room Temp
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Groups 3 12 On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
