What Is The Oxidation State Of S In H2so4
listenit
Apr 01, 2025 · 6 min read
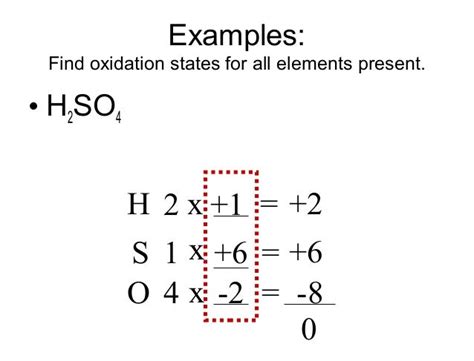
Table of Contents
What is the Oxidation State of S in H₂SO₄? A Deep Dive into Sulfur's Chemistry
Determining the oxidation state of an atom within a molecule is a fundamental concept in chemistry. It helps us understand the reactivity of the compound and predict its properties. This article will delve into the detailed calculation and explanation of the oxidation state of sulfur (S) in sulfuric acid (H₂SO₄), a crucial compound in various industrial processes and naturally occurring in acid rain. We will also explore the broader implications of oxidation states and their relevance in understanding chemical reactions.
Understanding Oxidation States
Before we tackle the specific case of H₂SO₄, let's establish a clear understanding of what oxidation states represent. The oxidation state, also known as oxidation number, is a hypothetical charge assigned to an atom in a molecule or ion, assuming that all bonds are completely ionic. This means we assign electrons to the more electronegative atom in each bond. While not a true charge, the oxidation state provides a valuable tool for:
- Balancing redox reactions: Oxidation states help us track electron transfer during chemical reactions, which are crucial for balancing redox equations.
- Predicting reactivity: The oxidation state offers insights into an atom's tendency to gain or lose electrons, impacting its chemical behavior.
- Understanding chemical bonding: It provides a simplified model for describing the distribution of electrons in a molecule.
Several rules guide the assignment of oxidation states:
- Free elements: The oxidation state of an atom in its elemental form is always 0. For example, the oxidation state of S in S₈ is 0.
- Monatomic ions: The oxidation state of a monatomic ion is equal to its charge. For instance, the oxidation state of Na⁺ is +1, and Cl⁻ is -1.
- Oxygen: Oxygen typically has an oxidation state of -2, except in peroxides (like H₂O₂) where it's -1 and in compounds with fluorine (like OF₂) where it's +2.
- Hydrogen: Hydrogen typically has an oxidation state of +1, except in metal hydrides (like NaH) where it's -1.
- Sum of oxidation states: The sum of the oxidation states of all atoms in a neutral molecule must equal zero. In a polyatomic ion, the sum equals the charge of the ion.
Calculating the Oxidation State of Sulfur in H₂SO₄
Now, let's apply these rules to determine the oxidation state of sulfur (S) in sulfuric acid (H₂SO₄). We'll follow a step-by-step approach:
-
Oxidation state of Hydrogen: Each hydrogen atom (H) has an oxidation state of +1. Since there are two hydrogen atoms, their total contribution is +2.
-
Oxidation state of Oxygen: Each oxygen atom (O) has an oxidation state of -2. Since there are four oxygen atoms, their total contribution is -8.
-
Let 'x' represent the oxidation state of Sulfur: We need to determine the value of 'x' for the sulfur atom.
-
Applying the rule of sum: The sum of the oxidation states in a neutral molecule (H₂SO₄) must be zero. Therefore, we can write the equation:
(+2) + x + (-8) = 0
-
Solving for x: Solving this simple algebraic equation, we get:
x = +6
Therefore, the oxidation state of sulfur (S) in sulfuric acid (H₂SO₄) is +6.
Implications of the +6 Oxidation State of Sulfur in H₂SO₄
The +6 oxidation state of sulfur in H₂SO₄ has significant implications for its chemical properties and reactivity. This high oxidation state indicates that sulfur has lost six electrons, making it a strong oxidizing agent. This explains why sulfuric acid is such a powerful oxidizing agent in many chemical reactions.
Strong Oxidizing Agent
Sulfuric acid's oxidizing power is evident in its reactions with various metals and non-metals. For example, it readily oxidizes less reactive metals like copper (Cu) to copper(II) ions (Cu²⁺) and is reduced itself to sulfur dioxide (SO₂), where sulfur has an oxidation state of +4.
Dehydrating Agent
The +6 oxidation state also contributes to sulfuric acid's dehydrating properties. Its strong affinity for water allows it to remove water molecules from many compounds, a crucial role in many organic chemical syntheses.
Acidic Nature
The high oxidation state does not directly influence acidity, but the highly polar nature of the S=O bonds and the presence of highly electronegative oxygen atoms contribute to the strong acidic character of H₂SO₄. The release of protons (H⁺) leads to its highly acidic nature.
Sulfur's Variable Oxidation States
It's important to note that sulfur exhibits a wide range of oxidation states, from -2 (as in hydrogen sulfide, H₂S) to +6 (as in H₂SO₄). This versatility contributes to the diverse chemistry of sulfur and the many different compounds it forms. Understanding the factors influencing these oxidation states is crucial in predicting the behavior of sulfur-containing compounds.
Factors influencing Oxidation States
Several factors determine the oxidation state sulfur assumes in a given compound:
- Electronegativity: The electronegativity of the other atoms bonded to sulfur plays a significant role. More electronegative atoms will tend to draw electrons away from sulfur, resulting in higher oxidation states.
- Bonding: The type of bonds (single, double, or triple) formed by sulfur with other atoms affects the electron distribution and consequently the oxidation state.
- Steric Factors: The arrangement of atoms around sulfur influences the oxidation state; this is particularly relevant in larger molecules where steric hindrance can affect bond formation.
This variability allows sulfur to participate in numerous redox reactions and to form a wide array of compounds with diverse applications.
Importance of Oxidation State in Redox Reactions
The concept of oxidation state is indispensable when dealing with redox (reduction-oxidation) reactions. In these reactions, electrons are transferred between species. Oxidation involves the loss of electrons, leading to an increase in oxidation state, while reduction involves the gain of electrons, resulting in a decrease in oxidation state.
Understanding the changes in oxidation states allows us to:
- Balance redox equations: By tracking electron transfer, we can ensure that the number of electrons lost in oxidation equals the number of electrons gained in reduction.
- Predict reaction products: Knowing the potential oxidation states of the reacting species allows us to predict the likely products of the reaction.
- Analyze reaction mechanisms: Oxidation state changes provide insights into the steps involved in redox reactions, helping us understand the reaction mechanism.
Applications of H₂SO₄ and its Significance
Sulfuric acid (H₂SO₄) is a cornerstone of many industries due to its versatility and reactivity. Its applications are extensive, including:
- Fertilizer Production: A massive amount of sulfuric acid is used in the production of phosphate fertilizers, vital for agriculture.
- Petroleum Refining: It plays a critical role in refining crude oil, removing impurities and facilitating the production of various petroleum products.
- Metal Processing: Used in the processing of many metals, including the purification of metals through leaching.
- Chemical Synthesis: A vital reagent in countless chemical syntheses, from manufacturing plastics to pharmaceuticals.
- Battery Production: Used in the production of lead-acid batteries, widely used in automobiles and other applications.
Conclusion
Determining the oxidation state of sulfur in sulfuric acid (H₂SO₄) is a straightforward application of fundamental chemical principles. The +6 oxidation state of sulfur reflects its strong oxidizing ability and contributes significantly to the compound's extensive applications. Understanding oxidation states is essential for comprehending chemical reactions, predicting reactivity, and analyzing complex chemical processes. The wide-ranging applications of sulfuric acid demonstrate the significant impact of sulfur chemistry on various aspects of modern society. Further exploration of sulfur chemistry, including its various oxidation states and their influence on the properties and reactivity of sulfur-containing compounds, provides valuable insights into the fundamental aspects of chemistry and the development of novel materials and technologies.
Latest Posts
Latest Posts
-
Lowest Common Denominator Of 7 And 8
Apr 02, 2025
-
What Is 21 30 As A Percent
Apr 02, 2025
-
How Hot Is 42 Degrees Celsius
Apr 02, 2025
-
How To Find The Mass Of Liquid
Apr 02, 2025
-
What Kingdom Does A Human Belong To
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation State Of S In H2so4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
