For Which Of The Mixtures Will Ag2so4 S Precipitate
listenit
Apr 06, 2025 · 5 min read
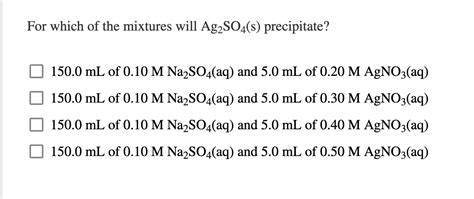
Table of Contents
Predicting Ag₂SO₄ Precipitation: A Comprehensive Guide
Silver sulfate (Ag₂SO₄) precipitation is a fascinating chemical phenomenon with implications across various fields, from analytical chemistry to industrial processes. Understanding the conditions under which Ag₂SO₄ precipitates is crucial for controlling reactions and achieving desired outcomes. This comprehensive guide delves into the factors influencing Ag₂SO₄ precipitation, providing a detailed analysis of different mixtures and the principles governing solubility equilibria.
Understanding Solubility and the Solubility Product Constant (Ksp)
Before exploring specific mixtures, let's establish a foundational understanding of solubility and its quantification. Solubility refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure. For sparingly soluble ionic compounds like Ag₂SO₄, solubility is often expressed using the solubility product constant (Ksp).
The Ksp is an equilibrium constant that represents the product of the concentrations of the constituent ions raised to their stoichiometric coefficients, each at saturation. For Ag₂SO₄, the dissolution equilibrium and Ksp expression are:
Ag₂SO₄(s) ⇌ 2Ag⁺(aq) + SO₄²⁻(aq)
Ksp = [Ag⁺]²[SO₄²⁻]
The Ksp value is a constant at a given temperature. A smaller Ksp indicates lower solubility, meaning less of the salt will dissolve before reaching saturation. Conversely, a larger Ksp indicates higher solubility.
Factors Affecting Ag₂SO₄ Precipitation
Several factors influence whether Ag₂SO₄ will precipitate from a given mixture:
-
Concentration of Ag⁺ and SO₄²⁻ ions: This is the most critical factor. If the product of the concentrations of Ag⁺ and SO₄²⁻ ions squared ([Ag⁺]²[SO₄²⁻]) exceeds the Ksp, the solution is supersaturated, and Ag₂SO₄ will precipitate until the ion product equals the Ksp, restoring equilibrium.
-
Temperature: The solubility of most ionic compounds increases with temperature. Therefore, increasing the temperature generally reduces the likelihood of Ag₂SO₄ precipitation, while decreasing the temperature increases the likelihood.
-
Common Ion Effect: The presence of a common ion (either Ag⁺ or SO₄²⁻) from another soluble salt in the solution significantly reduces the solubility of Ag₂SO₄. This is because the increased concentration of the common ion shifts the equilibrium to the left, favoring the formation of the solid precipitate. Adding a solution containing AgNO₃ or Na₂SO₄ will dramatically decrease the solubility of Ag₂SO₄.
-
pH: The pH of the solution can indirectly influence Ag₂SO₄ precipitation if other species in the mixture react with Ag⁺ or SO₄²⁻ ions. For instance, the presence of strong acids or bases can affect the concentration of these ions, potentially impacting precipitation.
-
Complex Ion Formation: If ligands are present that can form stable complexes with Ag⁺ ions, the effective concentration of free Ag⁺ ions will decrease, thus reducing the likelihood of Ag₂SO₄ precipitation.
-
Solvent: The nature of the solvent affects the solubility of Ag₂SO₄. While water is the most common solvent, using a different solvent could alter the solubility and hence the precipitation behavior.
Analyzing Different Mixtures for Ag₂SO₄ Precipitation
Let's analyze different scenarios to determine whether Ag₂SO₄ will precipitate. We'll use hypothetical concentrations and assume a constant temperature for simplicity. Remember, the actual Ksp value for Ag₂SO₄ needs to be obtained from a reliable source for accurate calculations. For the purpose of this example, let's assume Ksp (Ag₂SO₄) = 1.2 x 10⁻⁵.
Scenario 1: Mixing AgNO₃ and Na₂SO₄ solutions
Consider mixing 100 mL of 0.01 M AgNO₃ with 100 mL of 0.01 M Na₂SO₄.
-
Calculate the initial concentrations after mixing: The volume doubles, so the concentrations are halved: [Ag⁺] = 0.005 M and [SO₄²⁻] = 0.005 M.
-
Calculate the ion product (IP): IP = [Ag⁺]²[SO₄²⁻] = (0.005)²(0.005) = 1.25 x 10⁻⁷
-
Compare IP to Ksp: Since IP (1.25 x 10⁻⁷) < Ksp (1.2 x 10⁻⁵), the solution is unsaturated, and no precipitation occurs.
Scenario 2: Increasing the concentration of reactants
Now, let's increase the concentrations of AgNO₃ and Na₂SO₄. Consider mixing 100 mL of 0.1 M AgNO₃ with 100 mL of 0.1 M Na₂SO₄.
-
Calculate the initial concentrations after mixing: [Ag⁺] = 0.05 M and [SO₄²⁻] = 0.05 M.
-
Calculate the ion product (IP): IP = [Ag⁺]²[SO₄²⁻] = (0.05)²(0.05) = 1.25 x 10⁻⁴
-
Compare IP to Ksp: Now, IP (1.25 x 10⁻⁴) > Ksp (1.2 x 10⁻⁵). The solution is supersaturated, and Ag₂SO₄ will precipitate.
Scenario 3: Common Ion Effect
Let's consider adding a solution containing a common ion. Suppose we add 10 mL of 1 M Na₂SO₄ to the solution in Scenario 1.
-
Calculate the new concentration of SO₄²⁻: We'll need to account for the dilution. The new total volume is 210 mL. The moles of SO₄²⁻ from Na₂SO₄ are (0.01 L)(0.005 M) = 5 x 10⁻⁵ mol and from the additional Na₂SO₄ are (0.01 L)(1 M) = 0.01 mol. Total moles = 0.01005 mol. The new [SO₄²⁻] ≈ 0.048 M.
-
Calculate the new ion product: IP = (0.005)²(0.048) ≈ 1.2 x 10⁻⁶
-
Compare IP to Ksp: Even with the added sulfate, the IP is still less than the Ksp, so no significant precipitation will occur. However, the solubility of Ag₂SO₄ is noticeably reduced compared to Scenario 1.
Scenario 4: Influence of other ions and complex formation
If we introduce ligands that strongly complex with Ag⁺, such as ammonia (NH₃), the equilibrium will shift to favour the formation of the complex ion, [Ag(NH₃)₂]⁺. This will effectively reduce the concentration of free Ag⁺, making precipitation less likely, even if the sulfate concentration is high.
Conclusion
Predicting Ag₂SO₄ precipitation involves considering several factors, primarily the concentrations of Ag⁺ and SO₄²⁻ ions, temperature, the common ion effect, pH, and the potential formation of complex ions. By carefully calculating the ion product and comparing it to the Ksp, we can determine whether a given mixture will result in precipitation. It's crucial to remember that this is a simplified model; in real-world situations, other factors may come into play, requiring a more detailed analysis. Understanding these principles is fundamental for controlling chemical reactions and achieving desired outcomes in various chemical applications. The examples provided illustrate how subtle changes in concentrations and the presence of other ions can significantly impact whether Ag₂SO₄ will precipitate. Further investigations using different combinations of ions and conditions would provide more comprehensive insights into this important chemical phenomenon.
Latest Posts
Latest Posts
-
How Many Ounces Is Two Pints
Apr 07, 2025
-
Combination Of Chemical Symbols And Numbers To Represent A Substance
Apr 07, 2025
-
What Is 1 2 Minus 1 3
Apr 07, 2025
-
The Most Reactive Group In The Periodic Table
Apr 07, 2025
-
Which Of The Following Statements About Entropy Is True
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about For Which Of The Mixtures Will Ag2so4 S Precipitate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.